Difference between revisions of "LII:The Comprehensive Guide to Physician Office Laboratory Setup and Operation/The clinical environment"
Shawndouglas (talk | contribs) m (→The clinical laboratory: Renamed) |
Shawndouglas (talk | contribs) (Created as needed.) |
||
| (47 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
<div class="nonumtoc">__TOC__</div> | |||
<div align="center">-----Return to [[LII:The Comprehensive Guide to Physician Office Laboratory Setup and Operation|the beginning]] of this guide-----</div> | |||
{{The Comprehensive Guide to Physician Office Laboratory Setup and Operation/The clinical environment}} | |||
= | <div align="center">-----Go to [[LII:The Comprehensive Guide to Physician Office Laboratory Setup and Operation/Primary laboratory testing domains in the POL|the next chapter]] of this guide-----</div> | ||
==Citation information for this chapter== | |||
'''Chapter''': 1. The clinical environment | |||
'''Title''': ''The Comprehensive Guide to Physician Office Laboratory Setup and Operation'' | |||
'''Edition''': Second edition | |||
'''Author for citation''': Shawn E. Douglas | |||
'''License for content''': [https://creativecommons.org/licenses/by-sa/4.0/ Creative Commons Attribution-ShareAlike 4.0 International] | |||
'''Publication date''': June 2022 | |||
<!--Place all category tags here--> | |||
<! | |||
Latest revision as of 19:21, 1 June 2022
1. The clinical environment
The physician office laboratory (POL) is a clinical laboratory that is physician-, partnership-, or group-maintained, with the goal of diagnosing, preventing, and/or treating a disease or impairment in a patient as part of a physician practice. While definitions vary from state to state, this is a solid enough definition for broader purposes. Approximately 40 percent of all clinical laboratories in the U.S. are POLs according to Centers for Medicare and Medicaid Services statistics from May 2022.[1], suggesting they serve an important role within the current healthcare paradigm. However, there are nuances to (and evolution of) the POL that make it worthy of further discussion. This chapter addresses the clinical laboratory testing environment, with a focus on these POLs and how they differ from other clinical labs.
1.1 The POL as a clinical laboratory
The physician office laboratory (POL) is a type of clinical laboratory located in an ambulatory or outpatient care setting, usually in the physician office. A clinical laboratory specializes in testing specimens from human patients to assist with the diagnosis, treatment, or monitoring of a patient condition. That testing generally depends on one or more of three common methodologies to meet those goals: comparing the current value of a tested substance to a reference value, examining a specimen with microscopy, and/or detecting the presence of infection-causing pathogens.[2] The success of these methodologies is largely dependent upon the actions of laboratory directors, supervisors, pathologists, cytotechnologists, histotechnologists, and clinical laboratory assistants who perform and interpret analyses of patient specimens using one or more techniques.[3] Those methodologies and techniques also require a wide variety of instruments and equipment. A histotechnologist will require a microtome to prepare a specimen for an anatomical pathology examination, and blood chemistry analyses depend on sample tubes, centrifuges, and blood analyzers. More advanced clinical laboratories performing molecular diagnostics techniques will use specialty tools like fluorescence microscopes and spectrometers. And all that equipment must meet manufacturing, testing, and calibration standards to ensure the utmost accuracy of tests.[4]
However, the clinical environment of the POL is somewhat different than your average reference or diagnostic lab that receives, processes, and reports on specimens en masse. The POL is typically a smaller operation, performing simple laboratory testing that can produce useful diagnostic data cheaply and rapidly. Rather than performing advanced pathology procedures that require specific equipment and expertise, the POL typically focuses on blood chemistry, urinalysis, and other testing domains that don't require significant resources and provide rapid results. This can be seen in Centers for Medicare and Medicaid Services statistics reported in May 2022 that show 70.6 percent of non-exempt POLs in the U.S. are certified to provide Clinical Laboratory Improvement Amendments (CLIA)-waived tests[5], "simple tests with a low risk for an incorrect result."[6] These "simple tests" don't require advanced equipment and highly-trained physicians. Urinalysis reagent strips, influenza nasal swabs, and whole blood mononucleosis kits are all CLIA-waived testing devices that can be used by well-trained phlebotomists, nurses, or laboratory assistants.[7] Some POLs opt to provide more advanced testing services, however, with 17.5 percent of all non-exempt POLs holding provider performed microscopy (PPM) certificates to perform moderate-level CLIA testing.[5] This allows POLs to perform moderate complexity tests like urine sediment analysis and the determination of "the presence or absence of bacteria, fungi, parasites, or cellular elements" in a specimen.[8] However, the majority of POLs remain smaller and simpler than their diagnostic lab counterparts.
1.2 Good laboratory practices
As previously stated, the ultimate goal of the clinical laboratory—and by extension, the POL—is to test specimens from human patients to assist with the diagnosis, treatment, or monitoring of a patient condition. This, of course, requires accurate results to ensure the best result. In the 1970s, the U.S. Food and Drug Administration noted non-clinical laboratories in many cases conceived experiments poorly, failed to inform laboratory personnel of protocol, and didn't regard strict laboratory procedure to be necessary. This brought about the Good Laboratory Practice regulations in November 1976.[9][10] Clinical laboratories were not left out of this recognition of the need for improvements, however. Though the Clinical Laboratories Improvement Act of 1967 brought about some reforms to laboratory practices[11], the act wasn't doing enough by the mid-1980s. The regulations were revised and put into effect on October 31, 1988 as the Clinical Laboratory Improvement Amendments of 1988.[12] Known as "CLIA," the regulations have helped shape the policy and procedure of clinical laboratories of all types, including how training and experience is gauged and documented, reagents are prepared, and quality control is approached.
Today in the U.S., like any other clinical lab, physician office laboratories must follow good laboratory practices to ensure the best outcomes for its associated patients. These practices must be engaged in at every stage of the laboratory testing process. During the initial test ordering process, for example, lab personnel must review orders for accuracy and seek verification from the physician if there are any questions. Following order entry, staff should complete a requisition and explain all preparation procedures to the patient. When the patient arrives, staff should use appropriate procedures and containers to collect the specimen(s) from the patient. Processing of the specimen should include proper storage, preservation (if required), labeling, and transportation. The POL must run quality control tests prior to testing the patient sample to ensure instruments are properly calibrated and appropriate testing proficiency is met. After test completion, a laboratory report is printed and the physician notified. Patients should also be notified per the policy of the physician practice. Disposal of laboratory waste is also part of good laboratory practice, as is proper documentation in the patient record regarding testing and results.[2]
1.3 Laboratory safety
Like any other laboratory, safety in the clinical laboratory is of vital importance. Good safety practices ensure the specimen being tested does not get contaminated, and they also protect the person doing the testing from infection or other issues resulting from exposure.
Quality control guidelines and standards ensure procedures are followed and equipment is checked, lowering specimen contamination risk and improving the accuracy of test results. Laboratory safety guidelines assist professionals with managing risk from biohazards, chemical hazards, or physical hazards that may be present in the laboratory. The two U.S government agencies that primarily set safety guidelines are the Centers for Disease Control and Prevention (CDC) and the Occupational Safety and Health Administration (OSHA). CDC training involves learning about the chain of infection and standard precautions for infection control, while OSHA biohazard training involves the blood borne pathogen standard (BBPS) as well as the exposure control plans and guidelines that promote staff health and safety. OSHA also requires training to deal with chemical hazards in the laboratory.[13][2]
Laboratories of any size must also deal with physical hazards such as obstructions, electrical equipment, fires, floods, and earthquakes. Preparing for these possible hazards in some cases can be as simple as ensuring a box is not placed where someone walking could trip over it. OSHA has numerous guidelines related to the physical hazard training, including how to conduct a fire drill. Other beneficial preparatory activities include organizing and documenting clearly labeled chemical inventories, providing clear access to material safety data sheets (MSDS), enacting a hazard communication program, and providing training on OSHA adherence protocols.[13][2]
The POL is not exempt from these quality control and safety considerations simply because it's smaller and less sophisticated, however. It may not have the chemical stocks and testing hazards of a large diagnostic lab, but specimens must still be kept uncontaminated, and procedures for using even the simplest of CLIA-waived test devices must be followed. Biohazards are still generated and must be treated appropriately using work-practice controls, personal protective equipment, and engineering controls. This includes handling bleach (sodium hypochlorite), one of the most prevalent chemicals in labs[14], which must still be handled properly to ensure human safety and equipment longevity.[13][2]
1.4 Regulatory compliance: HIPAA and PPACA
Clinical laboratories must comply with the Health Insurance Portability and Accountability Act of 1996 (HIPAA). Among HIPAAs many goals is the desire to improve privacy and security protections for an individual's personal and identifying health information. As such, laboratories are required to implement measures that prevent unauthorized disclosure of and access to a patient's protected health information (PHI) in the laboratory. In the original implementation of HIPAA, this meant that laboratory staff were discouraged from giving laboratory test results to a patient without physician permission.[2] However, in February 2014, the Department of Health and Human Services wanted to encourage patients to take a more proactive approach to their own health care by giving them a mechanism to learn more about their own health. The HHS put into place an amendment to CLIA that became effective in April 2014, allowing patients to request laboratory results directly from a laboratory. Under the change, laboratories (including POLs) were required to give patients their laboratory results within 30 days of a written request by the patient or authorized agent, while still maintaining safeguards related to patient data and other sections of HIPAA.[15] This of course applies to the POL and their associated physician offices, which remain the second-most common violator of HIPAA privacy regulations.[16]
Another federal statute that impacts laboratory testing is the Patient Protection and Affordable Care Act (PPACA), signed in 2010 by President Barack Obama. When enacted, this law cut fees paid for laboratory testing and established accountable care organizations (ACOs). Both the cuts to the Medicare Clinical Laboratory Fee Schedule and the creation of ACOs initially posed challenges to the laboratory—especially in the physician office—as economic concerns were expected to cause a laboratory to no longer have incentive to offer some forms of testing.[17] However, the PPACA brought with it a beneficial transition from an incentivized volumetric approach to clinical testing (fee for service) to a preventative approach focused on quality patient outcomes (value-based service).[18] This healthcare outcome approach matched will with the growing demand for point-of-care testing (POCT), which "promotes these goals with rapid test results that providers can use to immediately inform patients of their condition or progress, and modify their treatment on-site."[19] And while POCT—particularly CLIA-waived testing—can happen in many clinical labs, from the hospital to the urgent care clinic, it has become a significant source of testing for the POL.[20]
1.5 Regulatory compliance: CLIA
In 1988, CLIA was passed as an amendment to the original 1967 legislature.[12] CLIA attempts to ensure the accuracy, reliability, and timeliness of test results regardless of where the test was performed. As part of this process, seven different criteria are used to gauge and assign one of three complexity levels to laboratory devices, assays, and examinations: high, moderate, and waived.[6][21] Clinical laboratories handling specimens originating from the U.S. and its territories must apply for a CLIA certificate that is appropriate for the type of testing it performs.
The POL largely conducts CLIA-waived tests, with 70.6 percent of all non-exempt POLs in the U.S. running on a CLIA certificate of waiver as of May 2022.[5] These tests are recognized as simple to perform with a low risk of erroneous results and include among others urinalysis for pregnancy and drugs of abuse, blood glucose and cholesterol tests, and fertility analysis. Despite the simplicity of a waived test, it "needs to be performed correctly, by trained personnel and in an environment where good laboratory practices are followed."[22] As such, CMS provided additional enforcement of labs with CLIA certificates of waiver in the 2010s, conducting on-site visits to approximately two percent of such labs to verify quality testing, regulatory compliance, and test appropriateness.[23]
CLIA-waived testing is not the only testing that goes on at a POL. In some cases, a POL may also offer moderate-level provider performed microscopy (PPM) testing (17.5 percent of all POLs as of May 2022[5]), depending on the office specialty.[2] To perform this type of testing in addition to waived testing, a PPM certificate is required.
For POLs exclusively conducting waived testing, anyone can be the laboratory director; however, some states have different requirements, so it is important the POL checks with their local regulatory body when hiring staff for the laboratory. POLs that also incorporate PPM testing have different requirements for directors, who "must meet specific education, training and experience under subpart M of the CLIA requirements."[24]
1.6 Point-of-care testing
The College of American Pathologists (CAP) defines POCT as "testing that is performed near or at the site of a patient with the result leading to a possible change in the care of the patient."[25] Historically this sort of testing was mundane due to the nature of the available methods; however, today these tests have advanced to include even limited forms of molecular diagnostics testing.[26] Like waived CLIA tests, POCT can also be performed by laboratory personnel. However, both personnel and patients (those who use testing devices at home) must be trained on how to use POCT devices in order to get the most accurate results.[27][28]
Some POCT devices are gradually allowing the patient to send data from their instruments—or even their mobile phones—directly to the physician office. However, this has historically not always a straightforward procedure. As the CAP noted in 2013 concerning POCT, "interoperability should be developed or expanded ... to provide better oversight and incorporation of results into the electronic medical record."[29] Multi-stage efforts towards "Meaningful Use" of electronic health records (EHRs) that are able to accept patient-generated data went into effect in the early to mid-2010s, followed by other pushes towards integrating POCT results with a wide variety of informatics systems.[30][31] However, interoperability and meaningful use of POCT data and EHRs still has work to do. As Labcorp's strategic director of clinical technology Adam Plotts notes, "[t]he main challenge is the way technology brings outside patient data into the provider's workflow."[32] During the COVID-19 pandemic, this has extended to at-home testing and the reporting of results over a mobile device.[33] However, from the perspective of the POL, these same at-home test kits can be used in the POL, and as long as results get properly documented—preferably in the patient's EHR record—POCT provides a better chance at timely patient outcomes.
1.7 Provider-performed microscopy testing
CLIA has approved some tests for the provider performed microscopy (PPM) level, a subcategory of the moderate complexity level. These tests must be of moderate complexity and require a microscope as the primary analysis tool, and they must be performed by a qualified physician or nurse practitioner in a set period of time, with limited handling of the specimen for the utmost accuracy.[34] Eligibility of PPM tests is determined by CMS, and those tests include wet mounted tissue examinations, semen analysis, certain mucous and nasal smears, and certain urinalyses.[35] (For the full list, consult the CMS-updated PDF file.)
These individual tests are useful to many POLs, though they have their own procedures and require the ability to focus and maintain the microscope at optimal performance level. Part of doing so is learning the structures of the microscope and how to use it. Since these procedures are performed by physicians or mid-level practitioners, the provider should be well trained in how to do this. They should be able to identify the parts of the microscope and understand how the lenses work. Knowledge of proper slide preparation is also vital, as improperly prepared slides can ruin an otherwise correctly performed PPMT procedure. In addition to an appropriate microscope, PPMT procedures require several other items, including immersion oil, lens paper, and tissue (lint free, soft).[2] Finally, though PPMT isn't regulated, the provider and laboratory personnel should carefully document quality assurance procedures for PPMT at regular intervals.[34] As noted previously, POLs that incorporate PPM testing have different requirements for laboratory directors than CLIA-waived labs, requiring a higher level of documented training and competence.[24]
1.8 CLIA market and industry trends
1.8.1 Clinical laboratory testing trends
With over 13 billion laboratory tests performed in the United States every year, laboratory testing is the highest volume medical activity in the country.[36][37] Laboratory testing influences approximately two-thirds of all medical decisions, and this testing often directs far more expensive care.[36] As these trends continue, the laboratory will likely become a coordinator for the patient and care team. The laboratory will serve as a facilitator for the patient and clinicians alike to receive not only test results but also education about those results. The laboratory will also assist the clinical team with test utilization, reporting those results into the EHR as well as maintaining them.[36]
Laboratory testing is in an upward trend, as seen with the growth of point-of-care testing and the patient-centered medical home (PCMH). Given the popularity of consumer products that track healthcare data, this trend should continue for years to come. One example of this is VeinViewer, which assists the phlebotomist with the location of veins and eliminates the painful process of sticking a patient more than once in an attempt to draw blood.[38] Other technologies are driving trends in this area, including in the testing domain, where for example Healthy.io (which acquired Scanadu/inui Health in 2020) offers a urinalysis strip than can be analyzed with a mobile app.[39][40]
1.8.2 POL testing trends
The third installment of research firm Kalorama Information's Physician Office Laboratory Markets reported the number of POLs performing CLIA-waived in vitro diagnostic (IVD) testing increased an average annual rate of 3.8 percent from 2005 to 2013, though "the number of POLs conducting moderate- and high-complexity testing under CLIA compliance or accreditation decreased by an annual average rate of 0.8 percent."[41] In mid- to late 2014, Kalorama estimated that POLs were conducting nine percent of all clinical IVD testing in the United States, also noting that "[n]ine out of the ten most-performed POL tests in the United States are CLIA-waived."[42]
As for what's being tested, the report noted that "[i]n the past five years, the most performed POL tests have changed little with the exception of some recently CLIA-waived infectious disease tests and vitamin D testing."[41] The study also found that many of the CLIA waivers granted in 2014 have been for tests in "highly competitive, established POL segments, such as drugs-of-abuse testing, routine clinical chemistries, urinalysis, hormone tests (including pregnancy), and dipstick urinalysis."[43] Many of those IVD tests were likely waived by regulation or cleared for home or over-the-counter use; however, a few of those tests had to go through a more rigorous process to become CLIA-waived.
Kalorama Information hasn't released a POL-specific report since 2014, but some information can be gleaned from other sources. In 2020, market research from Health Industry Distributors Association (HIDA) reported that POCT was outpacing overall diagnostic markets, and that molecular diagnostics is one of the fastest growing lab subspecialties.[44] This matches Thill's 2020 assessment that molecular diagnostics testing is becoming more readily available to the POL.[26] Thill also highlights the potential for PPM testing to make a comeback in the POL after nearly a decade of the percentage of labs performing PPM and other forms of moderate testing dropping. Writing for Repertoire magazine, Thill envisions those numbers slowly increasing again in the future, particularly given the rapid technological developments in molecular diagnostics testing, shifting some CLIA moderate molecular tests to waived, and other high-complexity molecular test to moderate. POLs wanting to perform more COVID-19 testing for their patients may also be a motivating factor to move up to CLIA moderate testing. This move to moderate may also be compelling to larger physician practices of five or more physicians wanting to conduct a higher throughput of both waived and moderate testing.[26]
1.8.3 Addition of the Dual 510(k) and CLIA Waiver by Application process
Originally an IVD test product not CLIA-waived by regulation or not cleared for home or over-the-counter use—even if it had clear advantages as a CLIA-waived product—would have to first be taken through the FDA 510(k) premarketing submission process which would then classify it as moderate or high complexity.[45] Then, if the product was viable as a moderate complexity device, the manufacturer would try to market and make money on their product and then go through the CLIA Waiver by Application process if they thought there were clear benefits and eligibility for CLIA-waived product status. By 2014, this process worked well enough for many manufacturers but frustrate a few who deemed their product non-viable to market unless it were CLIA-waived.[46]
Initially driven by the Medical Device User Fee Amendments of 2012 (MDUFA III)—an FDA attempt "to increase the efficiency of regulatory processes in order to reduce the time it takes to bring safe and effective medical devices to the U.S. market"[47]—the FDA sought to overhaul its Administrative Procedures for CLIA Categorization guidance documents, tracking mechanisms, and public CLIA database to improve the IVD and medical equipment regulation process. Among these changes, effective March 21, 2014, was the addition of the Dual 510(k) and CLIA Waiver by Application process, specifically for "that small subset of products that are not really viable if they are not waived."[46][48][49] The process is specific, requiring a pre-submission to receive FDA feedback first and be cleared for the Dual strategy. If approved, both the 510(k) and the CLIA Waiver by Application can be submitted at the same time.[46][45][50]
On December 17, 2014, rapid diagnostic manufacturer Quidel Corporation announced its Sofia Strep A+ Fluorescent Immunoassay, which became the first product to be approved through the Dual program[51], opening a potential gateway for other rapid diagnostic companies to fast-track their product through FDA and CLIA waiver approval. By 2020, more finalized reforms to the Dual program clarified the submission process, with the FDA stating, "Use of the dual 510(k) and CLIA waiver by application pathway is optional; however, FDA believes this pathway is in many instances the least burdensome and fastest approach for manufacturers to obtain a CLIA waiver and at the same time as 510(k) clearance."[52] The FDA released its finalized guidance Recommendations for Dual 510(k) and CLIA Waiver by Application Studies in February 2020[53], which helped clarify their stance.
1.8.4 More sophisticated CLIA-waived tests appear
Since the mid-2010, more sophisticated tests eligible for labs with a certificate of waiver have been appearing, further increasing same-day testing opportunities in POLs. For example, the first ever waived rapid screening test for syphilis was approved in the United States in December 2014, allowing physicians to make a preliminary diagnosis in as soon as 12 minutes. The product came about with the recognition of a the increasing number of primary and secondary syphilis cases in the U.S., nearly 55,000 new cases a year.[54] In 2015, reportedly almost four percent of all approved CLIA-waived tests were HIV tests, and of labs that offered CLIA-waived testing in general, 36 percent of them included at least one CLIA-waived HIV test.[55]
One of the biggest changes in the mid-2010s was the anticipation for waived molecular and nucleic acid-based testing. In an April 2015 interview with GenomeWeb, Cepheid chairman and CEO John Bishop stated the company would be "adding a sales force specifically for the CLIA market in early May" with expectations that the "market is actually going to step up overall test volumes as you move more broadly-disseminated."[56] Alere was also making a similar focus, though both companies were arguably heading up the charge towards a new potential trend in the CLIA-waived testing market: molecular and nucleic acid-based testing.
"I think the molecular tests are going to grab market share very rapidly ... and that's going to get even more emphasized with all of the programs now on antibiotic stewardship and more diagnostically directed use of therapeutics."[56] - John Bishop, Cepheid, Inc. chairman and CEO
"[W]e look forward to kind of changing the concept in the marketplace, [and showing] that you can get a highly sensitive, molecular result in minutes."[57] - K.C. McGrath, Alere, Inc. respiratory product manager
"We expect many other simple and accurate tests using nucleic acid-based technology to be developed in the near future. Once cleared by FDA, such tests can allow health care professionals to receive test results more quickly to inform further diagnostic and treatment decisions."[58] - Alberto Gutierrez, FDA director of the Office of In Vitro Diagnostics and Radiological Health
"I think traditional [polymerase chain reaction] assays will get better, faster, their boxes will get smaller, and you'll start to see a lot more point-of-care approved tests."[59] - Christine Ginocchio, BioMérieux vice president of global microbiology affairs
Molecular diagnostic tests had long been the domain of the full-fledged laboratory, even as companies like Cepheid made progress with sales of its molecular Flu Xpert test[56], rated by CLIA for moderate complexity labs.[60] With only roughly 29 percent of POLs running at some level of moderate complexity or better[5], however, molecular testing seemed largely out-of-reach for the POL. However, in January 2015, Alere and the FDA announced that the i Influenza A & B test had become the first ever nucleic acid-based test to receive CLIA-waived status, meaning it could be offered in POLs with a certificate of waiver.[60][58] Of course, not only were molecular tests beginning to make appearances for testing respiratory afflictions like influenza, but the COVID-19 pandemic thrust waived molecular testing into the spotlight full-force. As of April 2022, nearly 20 CLIA-waived COVID-19 molecular tests—some approved for home use—were available under emergency use authorization (EUA).[61] Molecular-based sexually transmitted infection (STI) testing has also seen an uptick[62], highlighting the growing sophistication of waived tests available to POLs. In another example, in the spring of 2021, Binx Health became the first to have a CLIA-waived molecular chlamydia and gonorrhea POCT on the market.[63]
1.8.5 Other players in the CLIA market
Other entities have applied their focus to the CLIA market as well. Take AmericanBio, a custom reagent manufacturer that announced in October 2014 that it was implementing a "CLIA Grade product line and CLIA Grade Custom Manufacturing Services," helping device manufacturers sort through "regulatory framework, validation needs, consistency, and quality driven metrics that will support their diagnostic tool," both traditional and molecular.[64] Seminars and webinars about getting medical devices CLIA-waived by market research companies like Research and Markets and government agencies like the National Institutes of Health became more prominent in the mid-2010s also.[65][66] And niche businesses like MRI Global have popped up, offering specialty regulatory, device, and test development services for the CLIA market.[67]
One other growing player in the CLIA market is the pharmacy-based laboratory. Since June 2011, POLs have lost ground to pharmacy-based labs, which have gone from making up 2.7 percent of all labs in June 2011[68] to 8.0 percent in May 2022.[1] Though these numbers were growing before the COVID-19 pandemic, it likely only helped boost the pharmacy lab and its efforts to perform lab testing.[69] Writing for 360 Dx, Adam Bonislawski notes that "[p]harmacy testing is done within pharmacies themselves, which are allowed to offer CLIA-waived tests provided they are registered as CLIA-waved laboratories."[69] With the previously mentioned EUAs on CLIA-waived COVID-19 tests, this further emboldened pharmacy labs to muscle in on POL territory to the point of making pharmacies only second to POLs in CLIA-waived testing. However, like POLs, the question of the reimbursement of these other CLIA-waived tests (see next section) remains, not to mention the question of whether or not the POL has the better advantage to providing service at the point of care.[69]
When examining the economics of the POL, four considerations leap to mind: billing, insurance reimbursements, profitability/sustainability, and return on investment (ROI). In truth, all these considerations are closely related to each other, with the goal of at least meeting operating costs while providing quality care to patients. However, plenty of challenges must be navigated along the way, from meeting state and federal regulatory requirements to billing properly. For the POL in particular, the practice must decide which tests to offer, finding balance between the most commonly ordered IVD tests—such as dipstick urinalysis, complete blood counts, and prothrombin time[70]—and those that will potentially see rapid revenue growth, including anemia tests, chronic inflammation tests, and the glycated hemoglobin (HbA1c) diabetic test.[71]
Determining ROI on diagnostic services isn't straightforward, however. In their 2020 paper published in British Journal of Healthcare Management, Price et al. note that ROI "is most applicable when both costs and benefits are quantifiable."[72] Quantifying the benefits of adding clinical laboratory testing to your physician office isn't always a straightforward process, to be sure. For example, "patient satisfaction" is more a qualitative measurement of ROI. Additionally challenges appear when trying to determine ROI when adding in technology-based tools like a laboratory information system (LIS), as there are usually multiple stakeholders involved in the overall value proposition. Price et al. add[72]:
The combination of the return on investment with the value proposition provides a framework for identifying the expectation of the benefits of a new test (or a technology) tailored to the contribution (and needs) of each of the stakeholders. It informs the business case for change, as well as becoming the benchmark for delivering that change. To assist with this task, there are various tools available, such as time-driven activity-based costing and patient level information costing systems ...
However, one of the major issues POL operators must deal with when considering cost/benefit analysis is healthcare reform and reimbursement changes. For example, until CMS intervened in 2010, physicians could bill Medicare per drug panel, meaning an 11-panel urine drug screen could be billed 11 times. CMS amended their rules in 2010 to prevent this type of unintended billing behavior, making drug testing less lucrative in general, especially for the POL.[73] Another example can be found in the Protecting Access to Medicare Act (PAMA), passed in April 2014. Though its original purpose was to delay Medicare payment cuts to physicians until March 2015, Medicare cuts targeting the clinical laboratory fee schedule in 2017 were said to pose an economic threat to small laboratories, including POLs. CMS was set to switch to market-based rate changes that researchers like Kalorama Information and organizations like the National Independent Laboratory Association (NILA) and the American Association for Clinical Chemistry (AACC) believed would adversely affect small laboratories.[41][74][75] Additionally problems came out of PAMA, with a non-representative sample of provider payment data skewing common test reimbursement abnormally lower.[76][77] This has led to much debate about what to do with PAMA. An April 2021 Medicare Payment Advisory Commission report suggested the use of a random sampling of labs' payment data to guide reimbursement, with associations like NILA and the American Clinical Laboratory Association (ACLA) appearing to support the change in 2022.[76]
The economics of data management, which tools to use, and what data to save represent additional considerations for the POL. "To improve clinical lab profitability in today's healthcare environment, it is essential for any practice to establish an ongoing process to produce data relevant to the management of its patient base," Medical Source, Inc. CEO Keith LaBonte said in 2011, also noting the importance of identifying relevant data and implementing effective processes for staff to collect and organize that data in data management systems.[78]
For more on estimating ROI for your POL, see the U.S. Agency for Healthcare Research and Quality's (AHRQ's) 2016 Toolkit for Using the AHRQ Quality Indicators section "Return on Investment Estimation".[79] While the estimation tools are targeted at hospitals, many of the same principles may apply to your POL.
1.10 Data management
The role of the laboratory is shifting both by internal forces, like the adoption of new technology, and external ones, such as consumerism, information availability, and the development of complicated data streams. Laboratory professionals are moving to a role that places them in the center of the data stream. As such, effective data collection and management is becoming more important than ever. This requires not only quality tools but also smooth, consistent workflows that can easily be followed and updated.
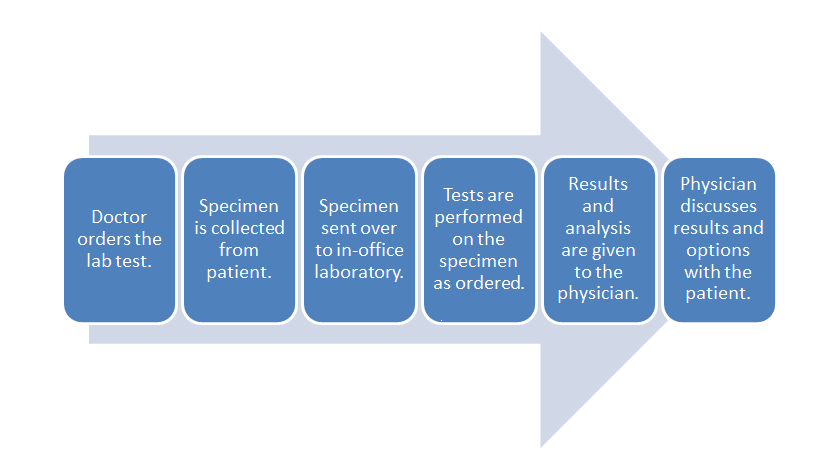
- POL workflow:
The above diagram shows the typical workflow for the POL. While managing this workflow is important, the data associated with it goes beyond ordinary practice function; the data can be used to assist with tracking population health for the patients or be combined with financial data such as billing or spending on supplies.
Other data streams may be generated outside this typical workflow as well. For example, the enactment of Meaningful Use Stage 3 by 2018 (the program was renamed from "Meaningful Use" to "Promoting Interoperability"[80][81]) meant EHRs had to be able to accept patient-generated data. This meant data coming from tracking tools like Fitbits or glucometers used by the patient had to be able to be accepted into an EHR.[82] This data, along with data from other POCT devices, aid the physician with diagnosis by giving them more data points to examine; however, this also requires the practice to have strong data management methods.
This area is a future frontier in the quest to use technology as a means of improving outcomes while driving costs down. The popularity of these patient devices and the trend towards using technology for healthcare purposes is drawing physician interest in using such devices for expanded monitoring of patients, with indicators supporting this trend on the development side.[82][83] The previously mentioned Healthy.io and its urinalysis strip than can be analyzed with a mobile app serves as one example.[39][40] Results can be stored and interpreted via the app, and that data can eventually make its way into a physician's compliant EHR. However, as Dihn-Le et al. pointed out in 2019, "[i]ncreased partnerships and opportunities between makers of these applications and health systems are necessary to reach high interoperability and streamlined communication between EHR platforms, patient devices, and providers."[83]
1.10.1 Data management tools and challenges
Laboratory data management for a POL has largely been done manually on paper or using office software like Excel. As technology has progressed, some of the larger physician office groups have adopted more sophisticated tools like the LIS, a software system that records, manages, and stores data for clinical laboratories. The LIS is able to connect to laboratory instruments, track orders, and record and store results. A modern LIS is also capable of integrating with an EHR to allow the results to be stored in the complete patient record. However, a full-featured LIS may be too much software for smaller POLs. Some companies like Relaymed have tried to solve this challenge by introducing cloud-based middleware-like solutions that help integrate POCT instruments and wearables with the EHR and test orders to make smoother POC workflows and data management in the POL.[84]
Other tools for connecting the lab with the physician office have emerged. Clinical information exchange (or health information exchange) systems have been designed to optimize workflows using application programming interfaces (APIs) and web services technology to connect systems.[85] (In fact, the previously mentioned Relaymed system is arguably in many ways a clinical information exchange system.) Computerized provider order entry (CPOE) systems are also increasingly being used. In many cases these systems are used for entering drug orders, but these systems can also be used to enter laboratory testing orders. This offers an opportunity for laboratory professionals to assist physicians with choosing the appropriate test.[86]
When done well, laboratory data management can allow physician offices to track data for multiple business processes as well as patient care. This is made easier when software systems and POCT instruments are integrated and the staff is trained in their use. As such, laboratory personnel should be part of the data management system decision making processes, especially given the introduction of patient-generated laboratory data. This also provides an opportunity for laboratory directors to get involved, as they are the ones ultimately responsible for compliance with regulatory issues, particularly since CLIA regulations hold laboratory directors responsible for compliance with data-reporting EHR requirements. Yes, the POL is not impacted by some of the challenges regarding CLIA requirements; however, it can still be impacted by the requirements to collect data from multiple sources and put that data into the patient record.[87] As most POLs are operating on a CLIA certificate of waiver and the requirements for the laboratory director are minimal, an air of complacency can evolve in the POL environment. However, a POL's laboratory director must focus on being proactively involved with data collection and management functions, as well as the EHR and LIS selection and familiarization process.
References
- ↑ 1.0 1.1 "Laboratories by Type of Facility" (PDF). Centers for Medicare and Medicaid Services. May 2022. https://www.cms.gov/Regulations-and-Guidance/Legislation/CLIA/downloads/factype.pdf. Retrieved 17 May 2022.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 Garrels, Marti; Oatis, Carol S. (2014). Laboratory and Diagnostic Testing in Ambulatory Care: A Guide for Healthcare Professionals (3rd ed.). Elsevier Health Sciences. pp. 368. ISBN 9780323292368. https://books.google.com/books?id=LM9sBQAAQBAJ. Retrieved 18 April 2022.
- ↑ "Careers in Pathology and Medical Laboratory Science" (PDF). American Society for Clinical Pathology. Archived from the original on 31 October 2015. https://web.archive.org/web/20151031084059/http://www.ascp.org/pdf/CareerBooklet.aspx. Retrieved 18 April 2022.
- ↑ "Laboratory Safety Standards". American National Standards Institute. 2022. https://webstore.ansi.org/industry/laboratory-safety. Retrieved 18 April 2022.
- ↑ 5.0 5.1 5.2 5.3 5.4 Centers for Medicare and Medicaid Services, Division of Laboratory Services (May 2022). "Enrollment, CLIA exempt states, and certification of accreditation by organization" (PDF). http://www.cms.gov/Regulations-and-Guidance/Legislation/CLIA/Downloads/statupda.pdf. Retrieved 17 May 2022.
- ↑ 6.0 6.1 Centers for Disease Control and Prevention (6 August 2018). "Clinical Laboratory Improvement Amendments (CLIA): Test complexities". https://www.cdc.gov/clia/test-complexities.html. Retrieved 18 April 2022.
- ↑ "CLIA - Clinical Laboratory Improvement Amendments - Currently Waived Analytes". U.S. Food and Drug Administration. 18 April 2022. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfClia/analyteswaived.cfm. Retrieved 18 April 2022.
- ↑ Kulczycki, Michael (1 April 2014). "Focusing on Provider-Performed Microscopy Procedure Requirements for Ambulatory Health Care". Ambulatory Buzz. The Joint Commission. Archived from the original on 01 March 2016. https://web.archive.org/web/20160301192508/http://www.jointcommission.org/musingsambulatory_patient_safety/focusing_provider_microscopy_procedure_req_ambulatory_health_care/. Retrieved 18 April 2022.
- ↑ Taylor, Jean M.; Stein, Gary C.; Weinberg, Sandy (2002). "Chapter 1: Historical Perspective". Good Laboratory Practice Regulations (3rd ed.). CRC Press. pp. 1–24. ISBN 9780203911082. https://books.google.com/books?id=50P7CAAAQBAJ&pg=PA1. Retrieved 18 April 2022.
- ↑ Seiler, Jürg P. (2006). "Chapter 1: What Is Good Laboratory Practice All About?". Good Laboratory Practice (2nd ed.). Springer Science & Business Media. pp. 1–58. ISBN 9783540282341. https://books.google.com/books?id=Hhj1sDFIlOYC&pg=PA1. Retrieved 18 April 2022.
- ↑ "Public Law 90-174" (PDF). United States Statutes at Large, Volume 81. 5 December 1967. https://www.govinfo.gov/content/pkg/STATUTE-81/pdf/STATUTE-81-Pg533.pdf. Retrieved 18 April 2022.
- ↑ 12.0 12.1 "Public Law 100-578" (PDF). United States Statutes at Large, Volume 102. 31 October 1988. https://www.govinfo.gov/content/pkg/STATUTE-102/pdf/STATUTE-102-Pg2903.pdf. Retrieved 18 April 2022.
- ↑ 13.0 13.1 13.2 Cox, Phyllis; Wilken, Danielle (2010). "Chapter 1: Safety in the Laboratory". Palko's Medical Laboratory Procedures (3rd ed.). McGraw-Hill Education. pp. 1–23. ISBN 9780073401959. https://books.google.com/books?id=6uWWPQAACAAJ. Retrieved 18 April 2022.
- ↑ "Examples of Common Laboratory Chemicals and their Hazard Class". National Institutes of Health, Office of Management. 27 November 2012. https://orf.od.nih.gov/EnvironmentalProtection/WasteDisposal/Pages/Examples+of+Common+Laboratory+ChemicalsandtheirHazardClass.aspx. Retrieved 18 April 2022.
- ↑ "CLIA Program and HIPAA Privacy Rule; Patients' Access to Test Reports" (PDF). Federal Register 79 (25): 7290–7316. 6 February 2014. https://www.govinfo.gov/content/pkg/FR-2014-02-06/pdf/2014-02280.pdf. Retrieved 18 April 2022.
- ↑ Robeznieks, A. (3 March 2021). "Common HIPAA violations physicians should guard against". American Medical Associations. https://www.ama-assn.org/practice-management/hipaa/common-hipaa-violations-physicians-should-guard-against. Retrieved 18 April 2022.
- ↑ Hughes, D.; Cammarata, B. (16 January 2014). "Clinical labs under ACA: Challenge and opportunity". Law360. https://www.law360.com/articles/500623/clinical-labs-under-aca-challenge-and-opportunity. Retrieved 18 April 2022.
- ↑ Laughlin, S. (22 October 2015). "The LIS, the healthcare market, and the POL". Medical Laboratory Observer. https://www.mlo-online.com/information-technology/lis/article/13008470/the-lis-the-healthcare-market-and-the-pol. Retrieved 18 April 2022.
- ↑ Rothenberg, I.Z. (9 July 2018). "Point of Care Testing (POCT): What’s New?". Physicians Office Resource. https://www.physiciansofficeresource.com/articles/laboratory/point-of-care-testing-poct-what-s-new/. Retrieved 18 April 2022.
- ↑ Rothenberg, I.Z. (1 July 2021). "The Increase in Waived Testing in the Physician Office". Physicians Office Resource. https://www.physiciansofficeresource.com/articles/finance/waived-testing/. Retrieved 18 April 2022.
- ↑ "CLIA Categorizations". U.S. Food and Drug Administration. 25 February 2020. https://www.fda.gov/medical-devices/ivd-regulatory-assistance/clia-categorizations. Retrieved 18 April 2022.
- ↑ "Waived Tests". Centers for Disease Control and Prevention. 3 September 2021. https://www.cdc.gov/labquality/waived-tests.html. Retrieved April 18, 2022.
- ↑ "Certificate of Waiver Laboratory Project". Centers for Medicare and Medicaid Services. 27 February 2014. Archived from the original on 02 January 2019. https://web.archive.org/web/20190102181225/https://www.cms.gov/Regulations-and-Guidance/Legislation/CLIA/Certificate_of_-Waiver_Laboratory_Project.html. Retrieved 18 April 2022.
- ↑ 24.0 24.1 "How to Apply for a CLIA Certificate, Including International Laboratories". Centers for Medicare and Medicaid Services. 1 December 2021. https://www.cms.gov/Regulations-and-Guidance/Legislation/CLIA/How_to_Apply_for_a_CLIA_Certificate_International_Laboratories. Retrieved 18 April 2022.
- ↑ Stiles, K. (2020). "Nebraska Public Health Laboratory Point of Care Waived Testing for Long Term Care Facilities". Nebraska Public Health Laboratory. https://www.nehca.org/wp-content/uploads/POC-Waived-Training-LTC.pdf. Retrieved 18 April 2022.
- ↑ 26.0 26.1 26.2 Thill, M. (October 2020). "Selling Moderate Complexity". Repertoire. https://repertoiremag.com/selling-moderate-complexity.html. Retrieved 18 April 2022.
- ↑ Kiechle, Frederick L.Main, Rhonda Ingram (2002). The Hitchhiker's Guide to Improving Efficiency in the Clinical Laboratory. American Association for Clinical Chemistry. pp. 132. ISBN 9781890883720. https://books.google.com/books?id=ud55aVHAiTQC. Retrieved 18 April 2022.
- ↑ "Point-of-Care Diagnostic Testing". Research Portfolio Online Reporting Tools. National Institutes of Health. 30 June 2018. https://web.archive.org/web/20190211145517/http://report.nih.gov/nihfactsheets/ViewFactSheet.aspx?csid=112. Retrieved 18 April 2022.
- ↑ "Point Of Care Testing". College of American Pathologists. September 2013. http://www.cap.org/apps//cap.portal?_nfpb=true&cntvwrPtlt_actionOverride=%2Fportlets%2FcontentViewer%2Fshow&_windowLabel=cntvwrPtlt&cntvwrPtlt{actionForm.contentReference}=policies%2Fpolicy_appII.html. [dead link]
- ↑ Futrell, K. (22 June 2017). "Looking at POCT through a new 'value' lens". Medical Laboratory Observer. https://www.mlo-online.com/continuing-education/article/13009205/looking-at-poct-through-a-new-value-lens. Retrieved 18 April 2022.
- ↑ Dhawan, Atam P. (2018). "Editorial Trends and Challenges in Translation of Point-of-Care Technologies in Healthcare". IEEE Journal of Translational Engineering in Health and Medicine 6: 1–8. doi:10.1109/JTEHM.2018.2866162. ISSN 2168-2372. PMC PMC6225954. PMID 30430044. https://ieeexplore.ieee.org/document/8485509/.
- ↑ Plotts, A. (21 September 2021). "Creating Meaningful Interoperability for Patient Healthcare Records". Labcorp. https://www.labcorp.com/unique-perspectives/blog/creating-meaningful-interoperability-patient-healthcare-records. Retrieved 20 April 2022.
- ↑ Juluru, K.; Weitz, A.; Fleurence, R.L. et al. (11 February 2022). "Reporting COVID-19 Self-Test Results: The Next Frontier" (in en). Health Affairs. doi:10.1377/forefront.20220209.919199. http://www.healthaffairs.org/do/10.1377/forefront.20220209.919199/full/.
- ↑ 34.0 34.1 "Waived and Provider Performed Microscopy (PPM) Tests". American Academy of Family Physicians. https://www.aafp.org/family-physician/practice-and-career/managing-your-practice/clia/waived-ppm-tests.html. Retrieved 20 April 2022.
- ↑ "Provider-performed Microscopy Procedures" (PDF). Centers for Medicare and Medicaid Services. https://www.cms.gov/Regulations-and-Guidance/Legislation/CLIA/downloads/ppmplist.pdf. Retrieved 20 April 2022.
- ↑ 36.0 36.1 36.2 "Integrating Laboratories Into the PCMH Model of Health Care Delivery: A COLA White Paper" (PDF). 2014. p. 3–4. http://www.cola.org/wp-content/uploads/2015/06/COLA_14194-PCMH-Whitepaper.pdf. Retrieved 20 April 2022.
- ↑ "Strengthening Clinical Laboratories". Centers for Disease Control and Prevention. 15 November 2018. https://www.cdc.gov/csels/dls/strengthening-clinical-labs.html. Retrieved 20 April 2022.
- ↑ Goyal, Nidhi (4 April 2015). "VeinViewer Means No More Poking People Relentlessly to Locate Veins". Industry Tap. https://www.industrytap.com/veinviewer-means-no-poking-people-relentlessly-locate-veins/27706. Retrieved 20 April 2022.
- ↑ 39.0 39.1 Buhr, Sarah (18 February 2015). "Scanadu’s New Pee Stick Puts The Medical Lab On Your Smartphone". Tech Crunch. Yahoo. https://techcrunch.com/2015/02/18/scanadus-new-pee-stick-puts-the-medical-lab-on-your-smartphone/. Retrieved 20 April 2022.
- ↑ 40.0 40.1 "About Us". Healthy.io. https://healthy.io/about-us. Retrieved 20 April 2022.
- ↑ 41.0 41.1 41.2 Park, Richard (13 October 2014). "Examining the Physician Office Lab Market: Growth and Reimbursement". Medical Design Technology. Advantage Business Media. Archived from the original on 14 September 2015. https://web.archive.org/web/20150915023725/http://www.mdtmag.com/blog/2014/10/examining-physician-office-lab-market-growth-and-reimbursement. Retrieved 01 June 2015.
- ↑ "How and Where IVD Will Find Growth in the Global POL Market – Part 2". Kalorama Information. November 2014. Archived from the original on 17 April 2015. https://web.archive.org/web/20150417204832/http://www.kaloramainformation.com/article/2014-11/How-and-Where-IVD-Will-Find-Growth-Global-POL-Market-%E2%80%93-Part-2. Retrieved 20 April 2022.
- ↑ Park, Richard (29 September 2014). "Examining the Physician Office Lab Market: CLIA and Technology". Medical Design Technology. Advantage Business Media. Archived from the original on 15 September 2015. https://web.archive.org/web/20150915023715/http://www.mdtmag.com/blog/2014/09/examining-physician-office-lab-market-clia-and-technology. Retrieved 20 April 2022.
- ↑ Health Industry Distributors Association (October 2020). "2020 U.S. Laboratory Market Report". Research and Markets. https://www.researchandmarkets.com/reports/5178165/2020-u-s-laboratory-market-report. Retrieved 20 April 2022.
- ↑ 45.0 45.1 "Administrative Procedures for CLIA Categorization: Guidance for Industry and Food and Drug Administration Staff" (PDF). U.S. Department of Health and Human Services. 12 March 2014. http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm070889.pdf. Retrieved 01 June 2015.
- ↑ 46.0 46.1 46.2 Wirt, Tammy (24 March 2014). "NWS HHS FDA CRDH" (PDF). U.S. Food and Drug Administration. Archived from the original on 07 February 2019. https://web.archive.org/web/20190207182128/http://www.fda.gov/downloads/Training/CDRHLearn/UCM390761.pdf. Retrieved 27 May 2022.
- ↑ "Fact Sheet: Medical Device User Fee Amendments of 2012". U.S. Food and Drug Administration. 3 August 2012. Archived from the original on 26 January 2015. https://web.archive.org/web/20150126091504/http://www.fda.gov/RegulatoryInformation/Legislation/FederalFoodDrugandCosmeticActFDCAct/SignificantAmendmentstotheFDCAct/FDASIA/ucm313695.htm. Retrieved 27 May 2022.
- ↑ Mullen, Allyson B. (19 March 2014). "FDA Issues Revised Final Guidance Regarding Administrative Procedures for CLIA Categorization". FDA Law Blog. Hyman, Phelps & McNamara, P.C. https://www.thefdalawblog.com/2014/03/fda-issues-revised-final-guidance-regarding-administrative-procedures-for-clia-categorization/. Retrieved 27 May 2022.
- ↑ Rath, Prakash (5 March 2014). "Administrative Changes to FDA’s CLIA Categorization Program" (PDF). U.S. Food and Drug Administration. Archived from the original on 08 February 2017. https://web.archive.org/web/20170208064913/https://ftp.cdc.gov/pub/CLIAC_meeting_presentations/Pdf/Addenda/cliac0314/04_Rath_Admin%20Changes%20to%20FDA's%20CLIA%20Program.pdf. Retrieved 27 May 2022.
- ↑ "CLIA Waiver by Application". U.S. Food and Drug Administration. 2 May 2022. https://www.fda.gov/medical-devices/ivd-regulatory-assistance/clia-waiver-application. Retrieved 27 May 2022.
- ↑ "Quidel Receives Simultaneous FDA Clearance And CLIA Waiver For Its Sofia® Strep A+ Fluorescent Immunoassay (FIA) Via The FDA's New Dual Submission Program". Med Device Online. VertMarkets, Inc. 17 December 2014. https://www.meddeviceonline.com/doc/quidel-receives-simultaneous-fda-clearance-and-clia-waiver-0001. Retrieved 27 May 2022.
- ↑ Mezher, M. (25 February 2020). "FDA Finalizes Guidances on CLIA Waiver Applications, 510(k) Dual Submissions". Regulatory Affairs Professionals Society. https://www.raps.org/news-and-articles/news-articles/2020/2/fda-finalizes-guidances-on-clia-waiver-application. Retrieved 20 April 2022.
- ↑ "Recommendations for Dual 510(k) and CLIA Waiver by Application Studies". U.S. Food & Drug Administration. February 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/recommendations-dual-510k-and-clia-waiver-application-studies. Retrieved 27 May 2022.
- ↑ "Trinity Biotech Announces CLIA Waiver of Rapid Syphilis Test". GlobeNewswire, Inc. 16 December 2014. https://www.globenewswire.com/news-release/2014/12/16/691813/10112606/en/Trinity-Biotech-Announces-CLIA-Waiver-of-Rapid-Syphilis-Test.html. Retrieved 20 April 2022.
- ↑ "HIV testing - prevalent CLIA waived tests". Percepta Associates, Inc. 12 March 2015. https://www.perceptaassociates.com/hiv-testing-clia-waived-tests/. Retrieved 20 April 2022.
- ↑ 56.0 56.1 56.2 Johnson, Madeleine (26 April 2015). "Cepheid Sees 'Significant Opportunity' in Menu Expansion, CLIA Market". GenomeWeb. Genomeweb LLC. https://www.genomeweb.com/business-news/cepheid-sees-significant-opportunity-menu-expansion-clia-market. Retrieved 20 April 2022.
- ↑ Johnson, Madeleine (19 June 2014). "With FDA-Cleared Flu Test, Alere Launches Isothermal MDx Platform for US Clinical Market". GenomeWeb. Genomeweb LLC. https://www.genomeweb.com/pcr/fda-cleared-flu-test-alere-launches-isothermal-mdx-platform-us-clinical-market. Retrieved 20 April 2022.
- ↑ 58.0 58.1 Seiffert, Don (12 January 2015). "FDA waiver of Alere's flu test poses threat to market leader Cepheid". Boston Business Journal. American City Business Journals. https://www.bizjournals.com/boston/blog/bioflash/2015/01/fda-waiver-of-aleres-flu-test-poses-threat-to.html. Retrieved 20 April 2022.
- ↑ Johnson, Madeleine (30 April 2015). "Q&A: BioMérieux's Christine Ginocchio Chats About Her Clinical Lab Career Arc, Syndromic PCR Panels". GenomeWeb. Genomeweb LLC. https://www.genomeweb.com/pcr/qa-biom-rieuxs-christine-ginocchio-chats-about-her-clinical-lab-career-arc-syndromic-pcr-panels. Retrieved 20 April 2022.
- ↑ 60.0 60.1 Johnson, Madeleine (12 January 2015). "With CLIA Waiver and Widespread Flu, Alere Ramps Up Molecular Test Production". GenomeWeb. Genomeweb LLC. https://www.genomeweb.com/pcr/clia-waiver-and-widespread-flu-alere-ramps-molecular-test-production. Retrieved 20 April 2022.
- ↑ "In Vitro Diagnostics EUAs - Molecular Diagnostic Tests for SARS-CoV-2". U.S. Food and Drug Administration. 18 April 2022. https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/in-vitro-diagnostics-euas-molecular-diagnostic-tests-sars-cov-2. Retrieved 20 April 2022.
- ↑ Larkin, P.M.K.; Garner, O.B. (1 July 2020). "Molecular Point-of-Care Testing in Clinical Laboratories". Clinical Laboratory News. American Association for Clinical Chemistry. https://www.aacc.org/cln/articles/2020/july/molecular-point-of-care-testing-in-clinical-laboratories. Retrieved 20 April 2022.
- ↑ Peck, A.D. (28 April 2021). "FDA Grants CLIA Waiver Allowing First Test for Chlamydia and Gonorrhea to Be Performed at the Point of Care". Dark Daily. https://www.darkdaily.com/2021/04/28/fda-grants-clia-waiver-allowing-first-test-for-chlamydia-and-gonorrhea-to-be-performed-at-the-point-of-care/. Retrieved 20 April 2022.
- ↑ "AmericanBio expands product offerings, introduces exclusive CLIA Grade product line". AmericanBio, Inc. 17 October 2014. Archived from the original on 27 May 2015. https://web.archive.org/web/20150527162034/https://www.americanbio.com/news-events/americanbio-expands-product-offerings-introduces-exclusive-clia-grade-product-line1. Retrieved 20 April 2022.
- ↑ "How to get a CLIA Waiver for your Medical Device: One and a Half Day In-person Seminar 2015 (Newark, NJ)". Research and Markets. May 2015. Archived from the original on 27 May 2015. https://web.archive.org/web/20150527174014/http://www.researchandmarkets.com/reports/3215400/how-to-get-a-clia-waiver-for-your-medical-device. Retrieved 20 April 2022.
- ↑ [08 January 2020 ""CLIA Waivers: The What, The Why, and The How" Webinar"]. National Institutes of Health. 15 April 2015. Archived from the original on 27 May 2015. 08 January 2020. Retrieved 20 April 2022.
- ↑ "FDA Clearance Consultants for 510(k) Submission". MRI Global. https://www.mriglobal.org/fda-clearance-consultants-for-510k-submission/. Retrieved 20 April 2022.
- ↑ "Laboratories by Type of Facility" (PDF). Centers for Medicare and Medicaid Services. June 2011. Archived from the original on 10 April 2012. https://web.archive.org/web/20120410205217/https://www.cms.gov/Regulations-and-Guidance/Legislation/CLIA/downloads/factype.pdf. Retrieved 17 April 2022.
- ↑ 69.0 69.1 69.2 Bonislawski, A. (2 February 2022). "COVID-19 Accelerating Trend Toward Pharmacy-Based Testing". 360 Dx. https://www.360dx.com/clinical-lab-management/covid-19-accelerating-trend-toward-pharmacy-based-testing. Retrieved 27 May 2022.
- ↑ "Top 10 Tests In Physician Office Revealed". Kalorama Information. 10 December 2014. Archived from the original on 05 June 2015. https://web.archive.org/web/20150605181846/http://www.kaloramainformation.com/about/release.asp?id=3686. Retrieved 21 June 2022.
- ↑ "Report: Five Fastest-Growing Tests in Physician Office Labs". PR Newswire. 18 December 2014. https://www.prnewswire.com/news-releases/report-five-fastest-growing-tests-in-physician-office-labs-300010866.html. Retrieved 21 April 2022.
- ↑ 72.0 72.1 Price, Christopher P; McGinley, Patrick; John, Andrew St (2 June 2020). "What is the return on investment for laboratory medicine? The antidote to silo budgeting in diagnostics" (in en). British Journal of Healthcare Management 26 (6): 1–8. doi:10.12968/bjhc.2019.0075. ISSN 1358-0574. http://www.magonlinelibrary.com/doi/10.12968/bjhc.2019.0075.
- ↑ Collen, Mark (2012). "Profit-Driven Drug Testing". Journal of Pain & Palliative Care Pharmacotherapy (26): 13–17. doi:10.3109/15360288.2011.650358. https://www.academia.edu/7840929/Profit-driven_drug_testing. Retrieved 21 April 2022.
- ↑ "Recapping the 'Doc Fix' Act's Impacts on Medicare Lab Reimbursement". Kalorama Information. June 2014. Archived from the original on 02 June 2015. https://web.archive.org/web/20150602204259/http://www.kaloramainformation.com/article/2014-06/Recapping-Doc-Fix-Acts-Impacts-Medicare-Lab-Reimbursement. Retrieved 02 June 2015.
- ↑ Malone, Bill (1 June 2014). "A New Era for Lab Reimbursement". Clinical Laboratory News. American Association for Clinical Chemistry. https://www.aacc.org/cln/articles/2014/june/lab-reimbursement. Retrieved 21 April 2022.
- ↑ 76.0 76.1 Bonislawski, A. (21 December 2021). "Lab Industry Hoping for Permanent Fix to PAMA in 2022". 360 Dx. Archived from the original on 28 December 2021. https://web.archive.org/web/20211228004059/https://www.360dx.com/clinical-lab-management/lab-industry-hoping-permanent-fix-pama-2022. Retrieved 21 April 2022.
- ↑ Poggi, J. (February 2020). "PAMA Two Years Later". Repertoire. https://repertoiremag.com/pama-two-years-later.html. Retrieved 21 April 2022.
- ↑ "Return on Investment Estimation" (PDF). Toolkit for Using the AHRQ Quality Indicators - How to Improve Hospital Quality and Safety. 2016. https://www.ahrq.gov/sites/default/files/wysiwyg/professionals/systems/hospital/qitoolkit/combined/f1_combo_returnoninvestment.pdf. Retrieved 31 May 2022.
- ↑ Bresnick, J. (24 April 2018). "CMS Renames Meaningful Use to Highlight Interoperability Goals". Health IT Analytics. https://healthitanalytics.com/news/cms-renames-meaningful-use-to-highlight-interoperability-goals. Retrieved 21 April 2022.
- ↑ AAP Division of Quality (13 June 2019). "Meaningful use program renamed; stage 3 requirements revised". AAP News. American Academy of Pediatrics. https://publications.aap.org/aapnews/news/11909. Retrieved 21 April 2022.
- ↑ 82.0 82.1 McLeod, Pamela Scherer (19 March 2015). "Physicians Use Fitness Trackers to Monitor Patients in Real-time, Even as Developers Work to Incorporate Medical Laboratory Tests into the Devices". Dark Daily. Dark Intelligence Group, Inc. http://www.darkdaily.com/physicians-use-fitness-trackers-to-monitor-patients-in-real-time-even-as-developers-work-to-incorporate-medical-laboratory-tests-into-the-devices-528. Retrieved 14 April 2015.
- ↑ 83.0 83.1 Dinh-Le, Catherine; Chuang, Rachel; Chokshi, Sara; Mann, Devin (2019). "Wearable Health Technology and Electronic Health Record Integration: Scoping Review and Future Directions". JMIR mHealth and uHealth 7 (9). doi:10.2196/12861. ISSN 2291-5222. PMC 6746089. PMID 31512582. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6746089/.
- ↑ "An alternative to a Lab Information System (LIS)". Relaymed Blog. Relaymed. 30 November 2021. https://relaymed.com/blog/an-alternative-to-lab-information-system/. Retrieved 21 April 2022.
- ↑ Michel, Robert (30 March 2015). "How Medical Laboratories Help Physicians Overcome the Failure of Many EHR Systems to Support Effective Lab Test Ordering and Lab Result Reporting". DarkDaily.com. Dark Intelligence Group, Inc. http://www.darkdaily.com/how-medical-laboratories-help-physicians-overcome-the-failure-of-many-ehr-systems-to-support-effective-lab-test-ordering-and-lab-result-reporting-330.
- ↑ Sinard, John (2006). Practical Pathology Informatics: Demystifying Informatics for the Practicing Anatomic Pathologist. Springer Science & Business Media. pp. 412. ISBN 9780387280585. https://books.google.com/books?id=WerUyK618fcC. Retrieved 21 April 2022.
- ↑ Henricks, Walter H. (18 March 2015). "Accreditation and Regulatory Implications of Electronic Health Records for Laboratory Reporting". InsuranceNewsNet.com, Inc. Archived from the original on 07 August 2015. http://insurancenewsnet.com/oarticle/2015/03/18/accreditation-and-regulatory-implications-of-electronic-health-records-for-labor-a-606123.html. Retrieved 21 April 2022.
Citation information for this chapter
Chapter: 1. The clinical environment
Title: The Comprehensive Guide to Physician Office Laboratory Setup and Operation
Edition: Second edition
Author for citation: Shawn E. Douglas
License for content: Creative Commons Attribution-ShareAlike 4.0 International
Publication date: June 2022