Difference between revisions of "Journal:A sustainable approach for the reliable and simultaneous determination of terpenoids and cannabinoids in hemp inflorescences by vacuum-assisted headspace solid-phase microextraction"
Shawndouglas (talk | contribs) (Saving and adding more.) |
Shawndouglas (talk | contribs) (→Notes: Cats) |
||
| (8 intermediate revisions by the same user not shown) | |||
| Line 19: | Line 19: | ||
|download = [https://www.sciencedirect.com/science/article/pii/S2772582022000110/pdfft?md5=e3b57c4573ce08ea3420689b22d59047&pid=1-s2.0-S2772582022000110-main.pdf https://www.sciencedirect.com/science/article/pii/S2772582022000110/pdfft] (PDF) | |download = [https://www.sciencedirect.com/science/article/pii/S2772582022000110/pdfft?md5=e3b57c4573ce08ea3420689b22d59047&pid=1-s2.0-S2772582022000110-main.pdf https://www.sciencedirect.com/science/article/pii/S2772582022000110/pdfft] (PDF) | ||
}} | }} | ||
{{ombox | {{ombox | ||
| type = | | type = content | ||
| style = | |||
| style | | text = This article contains rendered mathematical formulae. You ''may'' require the [https://chrome.google.com/webstore/detail/tex-all-the-things/cbimabofgmfdkicghcadidpemeenbffn?hl=en TeX All the Things] plugin for Chrome or the [https://addons.mozilla.org/en-US/firefox/addon/native-mathml/ Native MathML] add-on and [https://developer.mozilla.org/en-US/docs/Web/MathML/Fonts fonts] for Firefox if they don't render properly for you. | ||
| text = This article | |||
}} | }} | ||
==Abstract== | ==Abstract== | ||
''[[Cannabis sativa]]'' L. is an intriguing plant that has been exploited since ancient times for recreational, medical, textile and food purposes. The plant's most promising bioactive constituents discovered so far belong to the [[terpenoid]] and [[cannabinoid]] classes. These specialized metabolites are highly concentrated in the plant aerial parts, and their chemical characterization is crucial to guarantee the safe and efficient use of the plant material irrespective of which use it is. | ''[[Cannabis sativa]]'' L. is an intriguing plant that has been exploited since ancient times for recreational, medical, textile and food purposes. The plant's most promising bioactive constituents discovered so far belong to the [[terpenoid]] and [[cannabinoid]] classes. These specialized metabolites are highly concentrated in the plant aerial parts, and their chemical characterization is crucial to guarantee the safe and efficient use of the plant material irrespective of which use it is. | ||
This study investigates for the first time the use of vacuum-assisted headspace solid-phase microextraction (Vac-HSSPME) as a [[Sample (material)|sample]] preparation process in an analytical protocol based on Vac-HSSPME combined to fast [[gas chromatography–mass spectrometry]] (GC-MS) analysis that aims at comprehensively | This study investigates for the first time the use of vacuum-assisted headspace solid-phase microextraction (Vac-HSSPME) as a [[Sample (material)|sample]] preparation process in an analytical protocol based on Vac-HSSPME combined to fast [[gas chromatography–mass spectrometry]] (GC-MS) analysis that aims at comprehensively characterizing both the terpenoid and cannabinoid profiles of ''[[Cannabis]]'' [[inflorescence]]s in a single step. The results proved that vacuum in the headspace should be preferred over atmospheric pressure conditions as it ensures the fast recovery of cannabinoid markers at relatively lower sampling temperatures (i.e., 90°C) that do not discriminate the most volatile fraction nor cause the formation of artifacts when the sampling time is minimized. | ||
'''Keywords''': ''Cannabis sativa'' inflorescences, vacuum-assisted headspace solid-phase microextraction, volatilome, terpenoids, cannabinoids | '''Keywords''': ''Cannabis sativa'' inflorescences, vacuum-assisted headspace solid-phase microextraction, volatilome, terpenoids, cannabinoids | ||
==Introduction== | ==Introduction== | ||
''[[Cannabis sativa]]'' L. currently can be considered one of the most studied plants due to its relevance in the illicit drug market and in the textile and food industry | ''[[Cannabis sativa]]'' L. currently can be considered one of the most studied plants due to its relevance in the illicit drug market and in the textile and food industry<ref>{{Cite journal |last=Pellati |first=Federica |last2=Brighenti |first2=Virginia |last3=Sperlea |first3=Johanna |last4=Marchetti |first4=Lucia |last5=Bertelli |first5=Davide |last6=Benvenuti |first6=Stefania |date=2018-10-14 |title=New Methods for the Comprehensive Analysis of Bioactive Compounds in Cannabis sativa L. (hemp) |url=http://www.mdpi.com/1420-3049/23/10/2639 |journal=Molecules |language=en |volume=23 |issue=10 |pages=2639 |doi=10.3390/molecules23102639 |issn=1420-3049 |pmc=PMC6222702 |pmid=30322208}}</ref>, as well as its potential medical usage. Whether the plant is intended for recreational purposes, fiber production ([[hemp]]), or [[Cannabis (drug)|medical use]] depends on the content of two major [[cannabinoid]]s in the aerial parts of the plant: the [[Psychoactive drug|psychoactive]] [[Tetrahydrocannabinol|(-)-''trans''-Δ<sup>9</sup>-tetrahydrocannabinol]] (Δ<sup>9</sup>-THC) and [[cannabidiol]] (CBD), the latter displaying several biological activities but not the psychotropic one.<ref>{{Cite journal |last=Appendino |first=G. |last2=Chianese |first2=G. |last3=Taglialatela-Scafati |first3=O. |date=2011-03-01 |title=Cannabinoids: Occurrence and Medicinal Chemistry |url=http://www.eurekaselect.com/openurl/content.php?genre=article&issn=0929-8673&volume=18&issue=7&spage=1085 |journal=Current Medicinal Chemistry |language=en |volume=18 |issue=7 |pages=1085–1099 |doi=10.2174/092986711794940888}}</ref><ref name=":0">{{Cite journal |last=Jin |first=Dan |last2=Henry |first2=Philippe |last3=Shan |first3=Jacqueline |last4=Chen |first4=Jie |date=2021-07-01 |title=Identification of Chemotypic Markers in Three Chemotype Categories of Cannabis Using Secondary Metabolites Profiled in Inflorescences, Leaves, Stem Bark, and Roots |url=https://www.frontiersin.org/articles/10.3389/fpls.2021.699530/full |journal=Frontiers in Plant Science |volume=12 |pages=699530 |doi=10.3389/fpls.2021.699530 |issn=1664-462X |pmc=PMC8283674 |pmid=34276749}}</ref> The illicit drug chemotype, also known as type I, contains an excess of Δ9-THC and a limited amount of CBD. Contrary to that is the cannabis chemotype used in manufacturing (i.e., industrial hemp or type III), where the ratio is reversed and Δ<sup>9</sup>-THC content cannot exceed 0.2%. Finally, the type II chemotype, which is used for medical purposes, is defined as having high mean contents of both CBD and Δ<sup>9</sup>-THC (i.e., Bedrocan: 22% THC, <1% CBD; Bediol: 6.5% THC, 8% CBD).<ref name=":0" /><ref>{{Cite book |last=European Monitoring Centre for Drugs and Drug Addiction |date=2018 |title=Cannabis legislation in Europe: An overview |url=https://data.europa.eu/doi/10.2810/930744 |publisher=Publications Office |place=LU |pages=1–30 |doi=10.2810/930744}}</ref><ref name=":1">{{Cite journal |last=Calvi |first=Lorenzo |last2=Pentimalli |first2=Daniela |last3=Panseri |first3=Sara |last4=Giupponi |first4=Luca |last5=Gelmini |first5=Fabrizio |last6=Beretta |first6=Giangiacomo |last7=Vitali |first7=Davide |last8=Bruno |first8=Massimo |last9=Zilio |first9=Emanuela |last10=Pavlovic |first10=Radmila |last11=Giorgi |first11=Annamaria |date=2018-02 |title=Comprehensive quality evaluation of medical Cannabis sativa L. inflorescence and macerated oils based on HS-SPME coupled to GC–MS and LC-HRMS (q-exactive orbitrap®) approach |url=https://linkinghub.elsevier.com/retrieve/pii/S0731708517325086 |journal=Journal of Pharmaceutical and Biomedical Analysis |language=en |volume=150 |pages=208–219 |doi=10.1016/j.jpba.2017.11.073}}</ref> | ||
Other than Δ<sup>9</sup>-THC and CBD, the plant may synthesize several specialised metabolites, including additional phytocannabinoids and [[terpene]]s, amongst others | Other than Δ<sup>9</sup>-THC and CBD, the plant may synthesize several specialised metabolites, including additional phytocannabinoids and [[terpene]]s, amongst others<ref name=":2">{{Cite journal |last=Andre |first=Christelle M. |last2=Hausman |first2=Jean-Francois |last3=Guerriero |first3=Gea |date=2016-02-04 |title=Cannabis sativa: The Plant of the Thousand and One Molecules |url=http://journal.frontiersin.org/Article/10.3389/fpls.2016.00019/abstract |journal=Frontiers in Plant Science |volume=7 |doi=10.3389/fpls.2016.00019 |issn=1664-462X |pmc=PMC4740396 |pmid=26870049}}</ref>, which are both produced by stalked glandular [[trichome]]s that are highly concentrated on female [[inflorescence]]s.<ref>{{Cite journal |last=Russo |first=Ethan B |date=2011-08 |title=Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects: Phytocannabinoid-terpenoid entourage effects |url=https://onlinelibrary.wiley.com/doi/10.1111/j.1476-5381.2011.01238.x |journal=British Journal of Pharmacology |language=en |volume=163 |issue=7 |pages=1344–1364 |doi=10.1111/j.1476-5381.2011.01238.x |pmc=PMC3165946 |pmid=21749363}}</ref> Phytocannabinoids are C21 compounds known as terpenophenolic compounds. They are produced by the plant in their acidic form, which under heating or during storage is [[Decarboxylation|decarboxylated]] into the active neutral form.<ref name=":2" /> At least 104 cannabinoids have been isolated so far.<ref name=":3">{{Cite journal |last=Jin |first=Dan |last2=Dai |first2=Kaiping |last3=Xie |first3=Zhen |last4=Chen |first4=Jie |date=2020-12 |title=Secondary Metabolites Profiled in Cannabis Inflorescences, Leaves, Stem Barks, and Roots for Medicinal Purposes |url=http://www.nature.com/articles/s41598-020-60172-6 |journal=Scientific Reports |language=en |volume=10 |issue=1 |pages=3309 |doi=10.1038/s41598-020-60172-6 |issn=2045-2322 |pmc=PMC7039888 |pmid=32094454}}</ref> Aside from Δ<sup>9</sup>-THC, CBD, [[cannabichromene]] (CBC), and [[cannabigerol]] (CBG), other predominant secondary metabolites are [[Tetrahydrocannabinolic acid|Δ<sup>9</sup>-tetrahydrocannabinolic acid]] (Δ<sup>9</sup>-THCA), [[cannabidiolic acid]] (CBDA), [[cannabichromenic acid]] (CBCA), and [[cannabigerolic acid]] (CBGA), which are the precursors of the formerly mentioned compounds. Other minor cannabinoids include [[Cannabinol|cannabinolic acid]] (CBNA) and Δ<sup>8</sup>THCA, which are artifacts of Δ<sup>9</sup>THCA, as well as [[Cannabielsoin|cannabielsoic acid]] (CBEA) and [[Cannabinodiol|cannabinodiolic acid]] (CBNDA), which derive from CBDA.<ref name=":4">{{Cite journal |last=Pavlovic |first=Radmila |last2=Panseri |first2=Sara |last3=Giupponi |first3=Luca |last4=Leoni |first4=Valeria |last5=Citti |first5=Cinzia |last6=Cattaneo |first6=Chiara |last7=Cavaletto |first7=Maria |last8=Giorgi |first8=Annamaria |date=2019-10-15 |title=Phytochemical and Ecological Analysis of Two Varieties of Hemp (Cannabis sativa L.) Grown in a Mountain Environment of Italian Alps |url=https://www.frontiersin.org/article/10.3389/fpls.2019.01265/full |journal=Frontiers in Plant Science |volume=10 |pages=1265 |doi=10.3389/fpls.2019.01265 |issn=1664-462X |pmc=PMC6822994 |pmid=31708938}}</ref> | ||
[[Terpene]]s are ''cannabis'''s most abundant specialized metabolites, including at least 120 identified terpenoids. | [[Terpene]]s are ''cannabis'''s most abundant specialized metabolites, including at least 120 identified terpenoids.<ref name=":3" /> Literature data suggest that varying pharmaceutical properties between different ''Cannabis'' varieties can be attributed to synergistic interactions, known as the "[[entourage effect]]," between cannabinoids and terpenes.<ref name=":4" /> A comprehensive qualitative characterization of both the cannabinoid and terpene profiles of the plant raw material is therefore of utmost importance not only to define its rational use (i.e., whether the plant under investigation was cultivated for fiber production, medical, or drug purposes) but also to guarantee the efficacy and safety of its potential pharmaceutical application. | ||
The most employed method of extraction of cannabinoids from plant raw material is [[wikipedia:Extraction (chemistry)|solid-liquid extraction]] (SLE) using [[ethanol]] or [[acetone]] as extracting [[solvent]]s due to their affinity and consequent high extracting efficiency for cannabinoids. | The most employed method of extraction of cannabinoids from plant raw material is [[wikipedia:Extraction (chemistry)|solid-liquid extraction]] (SLE) using [[ethanol]] or [[acetone]] as extracting [[solvent]]s due to their affinity and consequent high extracting efficiency for cannabinoids.<ref>{{Cite journal |last=Mastellone |first=Giulia |last2=Marengo |first2=Arianna |last3=Sgorbini |first3=Barbara |last4=Scaglia |first4=Federica |last5=Capetti |first5=Francesca |last6=Gai |first6=Francesco |last7=Peiretti |first7=Pier Giorgio |last8=Rubiolo |first8=Patrizia |last9=Cagliero |first9=Cecilia |date=2022-02-03 |title=Characterization and Biological Activity of Fiber-Type Cannabis sativa L. Aerial Parts at Different Growth Stages |url=https://www.mdpi.com/2223-7747/11/3/419 |journal=Plants |language=en |volume=11 |issue=3 |pages=419 |doi=10.3390/plants11030419 |issn=2223-7747 |pmc=PMC8838183 |pmid=35161400}}</ref><ref name=":5">{{Cite journal |last=Citti |first=Cinzia |last2=Braghiroli |first2=Daniela |last3=Vandelli |first3=Maria Angela |last4=Cannazza |first4=Giuseppe |date=2018-01 |title=Pharmaceutical and biomedical analysis of cannabinoids: A critical review |url=https://linkinghub.elsevier.com/retrieve/pii/S0731708517311895 |journal=Journal of Pharmaceutical and Biomedical Analysis |language=en |volume=147 |pages=565–579 |doi=10.1016/j.jpba.2017.06.003}}</ref> [[High-performance liquid chromatography]] (HPLC) and [[gas chromatography]] coupled to [[mass spectrometry]] (GC-MS) are the analytical techniques of choice for many qualitative and quantitative analyses.<ref name=":5" /> HPLC is usually employed when the acid and the neutral form of the investigated cannabinoids must be measured separately, while GC analyses enable the characterization of the total cannabinoid content (e.g. the combined amount of THC and THCA), as GC systems, by definition, work with high temperatures that lead unavoidably to the decarboxylation of cannabinoid acids.<ref name=":5" /> The total cannabinoid content is usually measured as it best represents the pharmacological activity of the material, unless differently stated by legislation.<ref>{{Cite book |date=2013-03-06 |title=Recommended Methods for the Identification and Analysis of Cannabis and Cannabis Products |url=https://www.un-ilibrary.org/content/books/9789210555890 |language=en |publisher=United Nations Office on Drugs and Crime |doi=10.18356/1e8e4f16-en |isbn=978-92-1-055589-0}}</ref> | ||
Thanks to their volatile nature, the isolation of terpenes—and in particular of mono- and [[sesquiterpene]]s—from plant raw material is straightforward, and their profiling can be performed by [[Headspace technology|headspace]] [[solid-phase microextraction]] (HSSPME) online combined to GC-MS analysis | Thanks to their volatile nature, the isolation of terpenes—and in particular of mono- and [[sesquiterpene]]s—from plant raw material is straightforward, and their profiling can be performed by [[Headspace technology|headspace]] [[solid-phase microextraction]] (HSSPME) online combined to GC-MS analysis.<ref name=":1" /> | ||
The recovery of cannabinoids from solid matrices by HSSPME is also feasible but requires long sampling times in combination to high sampling temperatures due to their low volatility and low tendency to escape to the headspace. In 2004, Lachenmeier ''et al.'' optimized a successful HSSPME method followed by on-coating derivatization of the cannabinoids with ''N''-methyl-''N''-trimethylsilyltrifluoroacetamide (MSTFA) for the extraction of cannabinoids from hemp food products using 90°C as sampling temperature and 30 minutes of extraction time | The recovery of cannabinoids from solid matrices by HSSPME is also feasible but requires long sampling times in combination to high sampling temperatures due to their low volatility and low tendency to escape to the headspace. In 2004, Lachenmeier ''et al.'' optimized a successful HSSPME method followed by on-coating derivatization of the cannabinoids with ''N''-methyl-''N''-trimethylsilyltrifluoroacetamide (MSTFA) for the extraction of cannabinoids from hemp food products using 90°C as sampling temperature and 30 minutes of extraction time<ref>{{Cite journal |last=Lachenmeier |first=Dirk W. |last2=Kroener |first2=Lars |last3=Musshoff |first3=Frank |last4=Madea |first4=Burkhard |date=2004-01-01 |title=Determination of cannabinoids in hemp food products by use of headspace solid-phase microextraction and gas chromatography?mass spectrometry |url=http://link.springer.com/10.1007/s00216-003-2268-4 |journal=Analytical and Bioanalytical Chemistry |volume=378 |issue=1 |pages=183–189 |doi=10.1007/s00216-003-2268-4 |issn=1618-2642}}</ref>, while in 2005, Ilias ''et al.'' showed that cannabinoid extraction should be performed at 150°C to maximize their recovery in short sampling times (e.g., five minutes).<ref name=":6">{{Cite journal |last=Ilias |first=Yara |last2=Rudaz |first2=Serge |last3=Mathieu |first3=Patrick |last4=Christen |first4=Philippe |last5=Veuthey |first5=Jean-Luc |date=2005-11 |title=Extraction and analysis of differentCannabis samples by headspace solid-phase microextraction combined with gas chromatography-mass spectrometry |url=https://onlinelibrary.wiley.com/doi/10.1002/jssc.200500130 |journal=Journal of Separation Science |language=en |volume=28 |issue=17 |pages=2293–2300 |doi=10.1002/jssc.200500130 |issn=1615-9306}}</ref> However, in more recent work, Czégény ''et al.'' investigated the effect of temperature on the composition of pyrolysis products of CBD in e-cigarettes. They tested different operating temperatures (250–400°C), and they proved that, depending on the temperature and atmosphere (i.e., inert of oxidative condition), 25–52% of CBD can be converted into other cannabinoids, amongst which Δ<sup>9</sup>-THC, Δ<sup>8</sup>-THC, cannabinol (CBN), and CBC are the predominant [[Pyrolysis|pyrolysates]].<ref name=":7">{{Cite journal |last=Czégény |first=Zsuzsanna |last2=Nagy |first2=Gréta |last3=Babinszki |first3=Bence |last4=Bajtel |first4=Ákos |last5=Sebestyén |first5=Zoltán |last6=Kiss |first6=Tivadar |last7=Csupor-Löffler |first7=Boglárka |last8=Tóth |first8=Barbara |last9=Csupor |first9=Dezső |date=2021-12 |title=CBD, a precursor of THC in e-cigarettes |url=http://www.nature.com/articles/s41598-021-88389-z |journal=Scientific Reports |language=en |volume=11 |issue=1 |pages=8951 |doi=10.1038/s41598-021-88389-z |issn=2045-2322 |pmc=PMC8076212 |pmid=33903673}}</ref> Even though when performing HSSPME it is usually unlikely to reach such extreme temperatures, the results of Czégény ''et al.'' suggest that (1) reduced sampling temperature should be preferred to obtain a truthful cannabinoid fingerprint profile in the plant raw material, and (2) CBD potential degradation should be investigated during the optimization of the sampling temperature. | ||
As thoroughly described by Psillakis ''et al.'' | As thoroughly described by Psillakis ''et al.''<ref>{{Cite journal |last=Psillakis |first=Elefteria |date=2017-09 |title=Vacuum-assisted headspace solid-phase microextraction: A tutorial review |url=https://linkinghub.elsevier.com/retrieve/pii/S0003267017307390 |journal=Analytica Chimica Acta |language=en |volume=986 |pages=12–24 |doi=10.1016/j.aca.2017.06.033}}</ref><ref>{{Cite journal |last=Psillakis |first=Elefteria |date=2020-09 |title=The effect of vacuum: an emerging experimental parameter to consider during headspace microextraction sampling |url=https://link.springer.com/10.1007/s00216-020-02738-x |journal=Analytical and Bioanalytical Chemistry |language=en |volume=412 |issue=24 |pages=5989–5997 |doi=10.1007/s00216-020-02738-x |issn=1618-2642}}</ref><ref>{{Cite journal |last=Psillakis |first=Elefteria |last2=Mousouraki |first2=Antonia |last3=Yiantzi |first3=Evangelia |last4=Kalogerakis |first4=Nicolas |date=2012-06 |title=Effect of Henry's law constant and operating parameters on vacuum-assisted headspace solid phase microextraction |url=https://linkinghub.elsevier.com/retrieve/pii/S002196731200708X |journal=Journal of Chromatography A |language=en |volume=1244 |pages=55–60 |doi=10.1016/j.chroma.2012.05.006}}</ref><ref>{{Cite journal |last=Psillakis |first=Elefteria |last2=Yiantzi |first2=Evangelia |last3=Sanchez-Prado |first3=Lucia |last4=Kalogerakis |first4=Nicolas |date=2012-09 |title=Vacuum-assisted headspace solid phase microextraction: Improved extraction of semivolatiles by non-equilibrium headspace sampling under reduced pressure conditions |url=https://linkinghub.elsevier.com/retrieve/pii/S0003267012001195 |journal=Analytica Chimica Acta |language=en |volume=742 |pages=30–36 |doi=10.1016/j.aca.2012.01.019}}</ref>, vacuum is a powerful experimental parameter to consider for increasing the extraction kinetic of semi-volatile compounds during the HSSPME process. This is because in the case of semi-volatiles and under non-equilibrium conditions, a reduced pressure inside the sample container decreases the resistance to mass transfer in the gas zone at the solid-headspace interface. As a consequence, higher extraction efficiencies for semi-volatile compounds can be achieved in shorter sampling times, and potentially at milder extraction temperatures.<ref name=":8">{{Cite journal |last=Capetti |first=Francesca |last2=Rubiolo |first2=Patrizia |last3=Bicchi |first3=Carlo |last4=Marengo |first4=Arianna |last5=Sgorbini |first5=Barbara |last6=Cagliero |first6=Cecilia |date=2020-05 |title=Exploiting the versatility of vacuum‐assisted headspace solid‐phase microextraction in combination with the selectivity of ionic liquid‐based GC stationary phases to discriminate Boswellia spp. resins through their volatile and semivolatile fractions |url=https://onlinelibrary.wiley.com/doi/10.1002/jssc.202000084 |journal=Journal of Separation Science |language=en |volume=43 |issue=9-10 |pages=1879–1889 |doi=10.1002/jssc.202000084 |issn=1615-9306}}</ref><ref>{{Cite journal |last=Pollo |first=Breno J. |last2=Romero-Orejón |first2=Katherine L. |last3=Marsaioli |first3=Anita J. |last4=Rosa |first4=Paulo T.V. |last5=Augusto |first5=Fabio |date=2022-02 |title=Vacuum-assisted headspace solid-phase microextraction and gas chromatography coupled to mass spectrometry applied to source rock analysis |url=https://linkinghub.elsevier.com/retrieve/pii/S2772582021000012 |journal=Advances in Sample Preparation |language=en |volume=1 |pages=100001 |doi=10.1016/j.sampre.2021.100001}}</ref> | ||
This study investigates the advantages and disadvantages of using vacuum-assisted headspace solid-phase microextraction (Vac-HSSPME) over HSSPME as a sample preparation process to be exploited in analytical protocols, aiming to comprehensively characterize both the terpene and cannabinoid profiles of ''Cannabis'' inflorescences in a single step while employing a total analysis system. | This study investigates the advantages and disadvantages of using vacuum-assisted headspace solid-phase microextraction (Vac-HSSPME) over HSSPME as a sample preparation process to be exploited in analytical protocols, aiming to comprehensively characterize both the terpene and cannabinoid profiles of ''Cannabis'' inflorescences in a single step while employing a total analysis system. | ||
| Line 61: | Line 59: | ||
Vac-HSSPME experiments were performed in the same commercial headspace 20 mL crimp vials but hermetically sealed with a stainless steel closure (provided by Prof. Elefteria Psillakis) having a hole that could tightly accommodate a Thermogreen LB-1 septum with half-hole (Supelco, Bellefonte, USA), through which the air evacuation step and the SPME sampling were performed. | Vac-HSSPME experiments were performed in the same commercial headspace 20 mL crimp vials but hermetically sealed with a stainless steel closure (provided by Prof. Elefteria Psillakis) having a hole that could tightly accommodate a Thermogreen LB-1 septum with half-hole (Supelco, Bellefonte, USA), through which the air evacuation step and the SPME sampling were performed. | ||
For the inflorescences, 10 mg of the pulverized sample were placed inside the vial, which was again stored at −18°C for one hour and then air-evacuated. | For the inflorescences, 10 mg of the pulverized sample were placed inside the vial, which was again stored at −18°C for one hour and then air-evacuated.<ref name=":8" /> The air-evacuation step was performed with a 22-gauge hypodermic needle sealed to a 5 mL syringe tightly secured to the tubing of a N 820.3 FT.18 (7 mbar ultimate vacuum) pumping unit manufactured by KNF Lab (Milan, Italy). The needle was inserted through the septum, and the vial was air-evacuated for one minute. The procedure was the same for HSSPME, while omitting the air evacuation step. | ||
For CBD standard sampling, 10 µL of the 1.0 mg mL<sup>−1</sup> solution were introduced through the closure septum after the air-evacuation step. For HSSPME experiments, the liquid sample was introduced into the vial by the open-vial technique. | For CBD standard sampling, 10 µL of the 1.0 mg mL<sup>−1</sup> solution were introduced through the closure septum after the air-evacuation step. For HSSPME experiments, the liquid sample was introduced into the vial by the open-vial technique.<ref>{{Cite book |last=Kolb |first=Bruno |last2=Ettre |first2=Leslie S. |date=2006 |title=Static headspace-gas chromatography: theory and practice |chapter=Chapter 3: The technique of head-space-gas chromatography |edition=2nd ed |publisher=Wiley |place=Hoboken, N.J |pages=45–116 |isbn=978-0-471-74944-8}}</ref> | ||
After sampling, the fiber was withdrawn and the SPME device moved to the GC-MS system for analysis. GC desorption lasted 10 minutes to minimize carry-over. | After sampling, the fiber was withdrawn and the SPME device moved to the GC-MS system for analysis. GC desorption lasted 10 minutes to minimize carry-over. | ||
| Line 80: | Line 78: | ||
Analyses were carried out under the following conditions. Temperatures: injector: 250 °C, transfer line: 270 °C, ion source: 200 °C; carrier gas: He; flow control mode: constant linear velocity; flow rate: 1.00 mL min<sup>−1</sup> (conventional column), 0.72 mL min<sup>−1</sup> (narrow bore column); injection mode: split; split ratio: 1:20. | Analyses were carried out under the following conditions. Temperatures: injector: 250 °C, transfer line: 270 °C, ion source: 200 °C; carrier gas: He; flow control mode: constant linear velocity; flow rate: 1.00 mL min<sup>−1</sup> (conventional column), 0.72 mL min<sup>−1</sup> (narrow bore column); injection mode: split; split ratio: 1:20. | ||
The MS was operated in electron ionization mode (EI) at 70 eV, scan rate: 666 u/s, mass range: 35–350 m/z. Temperature programs: (i) 50°C (one minute)// 3°C/min//250°C (five minutes) for conventional MEGA-%; (ii) 50°C (30 seconds) //7.2°C/min// 250°C (two minutes) for the narrow-bore column. The chromatographic conditions for the narrow-bore columns were obtained by translating the method parameters through the Agilent method translator software. | The MS was operated in electron ionization mode (EI) at 70 eV, scan rate: 666 u/s, mass range: 35–350 m/z. Temperature programs: (i) 50°C (one minute)// 3°C/min//250°C (five minutes) for conventional MEGA-%; (ii) 50°C (30 seconds) //7.2°C/min// 250°C (two minutes) for the narrow-bore column. The chromatographic conditions for the narrow-bore columns were obtained by translating the method parameters through the Agilent method translator software.<ref name=":9">{{Cite web |title=GC Calculators and Method Translation Software |url=https://www.agilent.com/en/support/gas-chromatography/gccalculators |publisher=Agilent Technologies, Inc. |accessdate=29 January 2022}}</ref> Identification was performed via comparisons of linear retention indices and mass spectra either with those of authentic standards, or with data stored in commercial<ref name=":10">{{Citation |last=Linstrom |first=Peter |date=1997 |title=NIST Chemistry WebBook, NIST Standard Reference Database 69 |url=https://webbook.nist.gov/chemistry/ |work=NIST Chemistry WebBook |language= |publisher=National Institute of Standards and Technology |doi=10.18434/t4d303 |access-date=29 January 2022}}</ref> and in-house libraries. | ||
==Results and discussion== | ==Results and discussion== | ||
Table S1 in the Supplementary materials provides the list of the target compounds together with their physicochemical properties (i.e., LogKow, boiling point, and vapor pressure). The mono- and sesquiterpene markers to be investigated were chosen according to the results of Jin ''et al.'', who comprehensively profiled reference specialized metabolites in ''Cannabis'' inflorescences, leaves, stem barks, and roots for the three ''Cannabis'' chemotypes (i.e., Type I, II and III). | Table S1 in the Supplementary materials provides the list of the target compounds together with their physicochemical properties (i.e., LogKow, boiling point, and vapor pressure). The mono- and sesquiterpene markers to be investigated were chosen according to the results of Jin ''et al.'', who comprehensively profiled reference specialized metabolites in ''Cannabis'' inflorescences, leaves, stem barks, and roots for the three ''Cannabis'' chemotypes (i.e., Type I, II and III).<ref name=":3" /> | ||
===Preliminary optimization of the fiber coating and chromatographic conditions=== | ===Preliminary optimization of the fiber coating and chromatographic conditions=== | ||
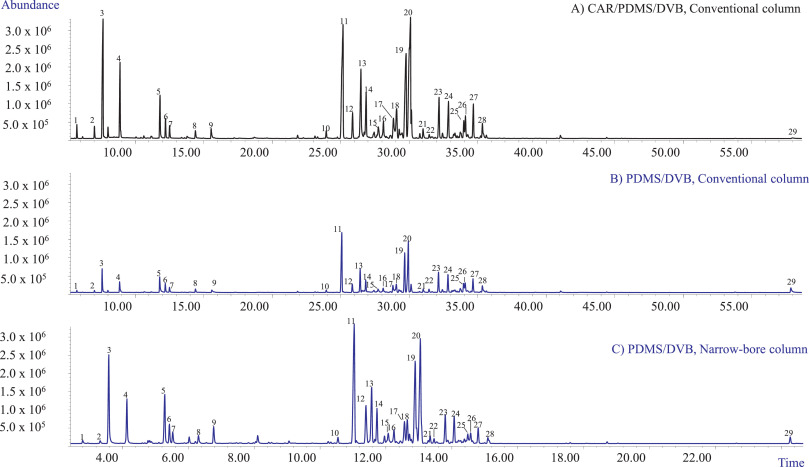
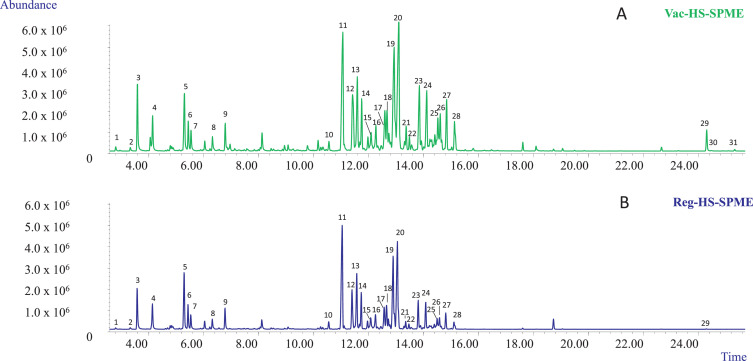
The first set of experiments aimed at selecting (1) the optimum fiber coating that could extract all the investigated analytes with acceptable sensitivity and (2) the best chromatographic conditions providing an acceptable resolution of all the investigated markers in a reasonable time for high-throughput analyses. In our study, two fiber coatings were tested: the PDMS/DVB and the DVB/CAR/PDMS fibers. Fig. 1A and B show the profiles obtained with the two investigated coatings when sampling 10 mg of matrix with HSSPME at 90°C for 30 minutes of extraction. The chromatographic analyses were performed employing a conventional 30 m × 0.25 mm ''d<sub>c</sub>'', 0.25 µm ''d<sub>f</sub>'' MEGA-5 column. Irrespective of the fiber coating, the most abundant compounds amongst the recovered mono- and sesquiterpenes were [[Myrcene|β-myrcene]] (3), [[Caryophyllene|trans-β-caryophyllene]] (11), and selina-3,7(11)-diene (20), while only one cannabinoid—CBD (29)—was recovered. In agreement with the results of Ilias ''et al.'' | The first set of experiments aimed at selecting (1) the optimum fiber coating that could extract all the investigated analytes with acceptable sensitivity and (2) the best chromatographic conditions providing an acceptable resolution of all the investigated markers in a reasonable time for high-throughput analyses. In our study, two fiber coatings were tested: the PDMS/DVB and the DVB/CAR/PDMS fibers. Fig. 1A and B show the profiles obtained with the two investigated coatings when sampling 10 mg of matrix with HSSPME at 90°C for 30 minutes of extraction. The chromatographic analyses were performed employing a conventional 30 m × 0.25 mm ''d<sub>c</sub>'', 0.25 µm ''d<sub>f</sub>'' MEGA-5 column. Irrespective of the fiber coating, the most abundant compounds amongst the recovered mono- and sesquiterpenes were [[Myrcene|β-myrcene]] (3), [[Caryophyllene|trans-β-caryophyllene]] (11), and selina-3,7(11)-diene (20), while only one cannabinoid—CBD (29)—was recovered. In agreement with the results of Ilias ''et al.''<ref name=":6" />, the PDMS/DVB fiber proved to be more efficient for the recovery of CBD compared to the triphasic fiber which, however, extracted a quantitative richer mono- and sesquiterpene profile. The PDMS/DVB fiber was chosen for the subsequent experiments because of its higher recovery of CBD while still providing an acceptable sensitivity for the investigated mono- and sesquiterpene metabolites. | ||
The employed conventional MEGA-5 column proved to separate the main selected markers with an acceptable resolution. The possibility to improve the analysis speed and to decrease the use of gas with a greener analytical protocol was then investigated. The chromatographic analysis was sped up by translating the method to a 15 m × 0.18 mm ''d<sub>c</sub>'', 0.18 µm ''d<sub>f</sub>'' column using the method translation approach. | The employed conventional MEGA-5 column proved to separate the main selected markers with an acceptable resolution. The possibility to improve the analysis speed and to decrease the use of gas with a greener analytical protocol was then investigated. The chromatographic analysis was sped up by translating the method to a 15 m × 0.18 mm ''d<sub>c</sub>'', 0.18 µm ''d<sub>f</sub>'' column using the method translation approach.<ref name=":9" /><ref>{{Cite journal |last=Bicchi |first=Carlo |last2=Blumberg |first2=Leonid |last3=Cagliero |first3=Cecilia |last4=Cordero |first4=Chiara |last5=Rubiolo |first5=Patrizia |last6=Liberto |first6=Erica |date=2010-02 |title=Development of fast enantioselective gas-chromatographic analysis using gas-chromatographic method-translation software in routine essential oil analysis (lavender essential oil) |url=https://linkinghub.elsevier.com/retrieve/pii/S0021967310000142 |journal=Journal of Chromatography A |language=en |volume=1217 |issue=9 |pages=1530–1536 |doi=10.1016/j.chroma.2010.01.003}}</ref><ref>{{Cite journal |last=Mazzucotelli |first=Maria |last2=Minteguiaga |first2=Manuel A. |last3=Sgorbini |first3=Barbara |last4=Sidisky |first4=Len |last5=Marengo |first5=Arianna |last6=Rubiolo |first6=Patrizia |last7=Bicchi |first7=Carlo |last8=Cagliero |first8=Cecilia |date=2020-01 |title=Ionic liquids as water-compatible GC stationary phases for the analysis of fragrances and essential oils: Quantitative GC–MS analysis of officially-regulated allergens in perfumes |url=https://linkinghub.elsevier.com/retrieve/pii/S0021967319309616 |journal=Journal of Chromatography A |language=en |volume=1610 |pages=460567 |doi=10.1016/j.chroma.2019.460567}}</ref> Fig. 1C reports the translated GC-MS profile of the investigated hemp inflorescences with the narrow-bore MEGA-5 column. The analysis time was reduced from 72.67 to 30.28 minutes, while maintaining the separation and the elution pattern of the investigated markers. | ||
| Line 98: | Line 96: | ||
|- | |- | ||
| style="background-color:white; padding-left:10px; padding-right:10px;" |<blockquote>'''Fig. 1''' HSSPME GC-MS profiles of the investigational hemp inflorescences obtained with different polymer coatings and chromatographic conditions. '''A)''' Polymer coating: CAR/PDMS/DVB; Column: conventional MEGA-5; GC-MS instrument: Agilent 6890 N GC coupled to a 5975 MSD. '''B)''' Polymer coating: PDMS/DVB; Column: conventional MEGA-5; GC-MS instrument: Agilent 6890 N GC coupled to a 5975 MSD. '''C)''' Polymer coating: PDMS/DVB; Column: narrow-bore MEGA-5; GC-MS instrument: Shimadzu QP2010-PLUS GC–MS system. GC-MS Analysis conditions: see experimental section. HSSPME parameters: sampling temperature 90°C, extraction time 30 minutes.<br /> <br />Legend: (1) α-Pinene, (2) β-Pinene, (3) β-Myrcene, (4) Limonene, (5) Linalool, (6) Fenchol, (7) cis-Pinene hydrate, (8) Borneol, (9) α-Terpineol, (10) α-Patchoulene, (11) trans-β-Caryophyllene, (12) trans-α-Bergamotene, (13) α-Humulene, (14) trans-β-Farnesene, (15) β-Selinene, (16) α-Selinene, (17) α-Farnesene, (18-19) Sesquiterpenes (MW 204), (20) Selina-3,7(11)-diene, (21) trans-Nerolidol, (22) Caryophyllene oxide, (23) Guaiol, (24) 10-epi-γ-Eudesmol, (25) β-Eudesmol, (26) α-Eudesmol, (27) Bulnesol, (28) α-Bisabolol, (29) Cannabidiol.</blockquote> | | style="background-color:white; padding-left:10px; padding-right:10px;" |<blockquote>'''Fig. 1''' HSSPME GC-MS profiles of the investigational hemp inflorescences obtained with different polymer coatings and chromatographic conditions. '''A)''' Polymer coating: CAR/PDMS/DVB; Column: conventional MEGA-5; GC-MS instrument: Agilent 6890 N GC coupled to a 5975 MSD. '''B)''' Polymer coating: PDMS/DVB; Column: conventional MEGA-5; GC-MS instrument: Agilent 6890 N GC coupled to a 5975 MSD. '''C)''' Polymer coating: PDMS/DVB; Column: narrow-bore MEGA-5; GC-MS instrument: Shimadzu QP2010-PLUS GC–MS system. GC-MS Analysis conditions: see experimental section. HSSPME parameters: sampling temperature 90°C, extraction time 30 minutes.<br /> <br />Legend: (1) α-Pinene, (2) β-Pinene, (3) β-Myrcene, (4) Limonene, (5) Linalool, (6) Fenchol, (7) cis-Pinene hydrate, (8) Borneol, (9) α-Terpineol, (10) α-Patchoulene, (11) trans-β-Caryophyllene, (12) trans-α-Bergamotene, (13) α-Humulene, (14) trans-β-Farnesene, (15) β-Selinene, (16) α-Selinene, (17) α-Farnesene, (18-19) Sesquiterpenes (MW 204), (20) Selina-3,7(11)-diene, (21) trans-Nerolidol, (22) Caryophyllene oxide, (23) Guaiol, (24) 10-epi-γ-Eudesmol, (25) β-Eudesmol, (26) α-Eudesmol, (27) Bulnesol, (28) α-Bisabolol, (29) Cannabidiol.</blockquote> | ||
|- | |||
|} | |||
|} | |||
===Vac-HSSPME and HSSPME=== | |||
Due to their relative high molecular weights and boiling points (e.g., CBD 428.52°C, 760 mmHg<ref>{{Cite web |date=18 January 2022 |title=EPI Suite - Estimation Program Interface |url=https://www.epa.gov/tsca-screening-tools/epi-suitetm-estimation-program-interface |publisher=U.S. Environmental Protection Agency |accessdate=02 February 2022}}</ref>) cannabinoids have a low tendency to escape to headspace and require high sampling temperatures to be recovered by HSSPME.<ref name=":6" /> However, in view of a reliable and comprehensive HSSPME method suitable for the simultaneous qualitative characterization of both the terpenoid and the cannabinoid fractions of ''Cannabis'' inflorescences, high sampling temperatures are not advisable for two reasons. First, they may decrease the distribution coefficient between the fiber and the headspace (i.e., K<sub>fs</sub>) of the most volatile components, reducing their recovery.<ref name=":11">{{Cite journal |last=Lord |first=Heather |last2=Pawliszyn |first2=Janusz |date=2000-07 |title=Evolution of solid-phase microextraction technology |url=https://linkinghub.elsevier.com/retrieve/pii/S0021967300005355 |journal=Journal of Chromatography A |language=en |volume=885 |issue=1-2 |pages=153–193 |doi=10.1016/S0021-9673(00)00535-5}}</ref> In addition, a high sampling temperature, especially when combined with relatively long extraction times, may induce decomposition of some compounds and/or creation of other components or artifacts.<ref>{{Cite journal |last=Risticevic |first=Sanja |last2=Lord |first2=Heather |last3=Górecki |first3=Tadeusz |last4=Arthur |first4=Catherine L |last5=Pawliszyn |first5=Janusz |date=2010-01 |title=Protocol for solid-phase microextraction method development |url=http://www.nature.com/articles/nprot.2009.179 |journal=Nature Protocols |language=en |volume=5 |issue=1 |pages=122–139 |doi=10.1038/nprot.2009.179 |issn=1754-2189}}</ref> According to the theory, a reduced pressure inside the headspace sample container increases the compounds' molecular diffusion coefficient in air and favors their vapor flux at the solid surface. As a result, reducing the total headspace pressure is an alternative and complementary strategy, compared to the adoption of high sampling temperatures, to speed up the extraction kinetic of semi-volatile compounds.<ref>{{Cite journal |last=Yiantzi |first=Evangelia |last2=Kalogerakis |first2=Nicolas |last3=Psillakis |first3=Elefteria |date=2015-08 |title=Vacuum-assisted headspace solid phase microextraction of polycyclic aromatic hydrocarbons in solid samples |url=https://linkinghub.elsevier.com/retrieve/pii/S0003267015007989 |journal=Analytica Chimica Acta |language=en |volume=890 |pages=108–116 |doi=10.1016/j.aca.2015.05.047}}</ref> | |||
In this study we compared the performances of Vac-HSSPME to those of HSSPME under different sampling temperatures (i.e., 150°C, 90°C, and 80°C) and extraction times (i.e., 5, 15, 30 minutes), and we explored whether Vac-HSSPME could be a more suitable sample preparation technique for the simultaneous characterization of both the terpenoid and cannabinoid profiles of ''Cannabis'' inflorescences. For the following discussion, CBD will be considered as representative of the plant cannabinoids, while β-myrcene and trans-β-caryophyllene will be considered representative of the mono- and sesquiterpene markers, respectively. | |||
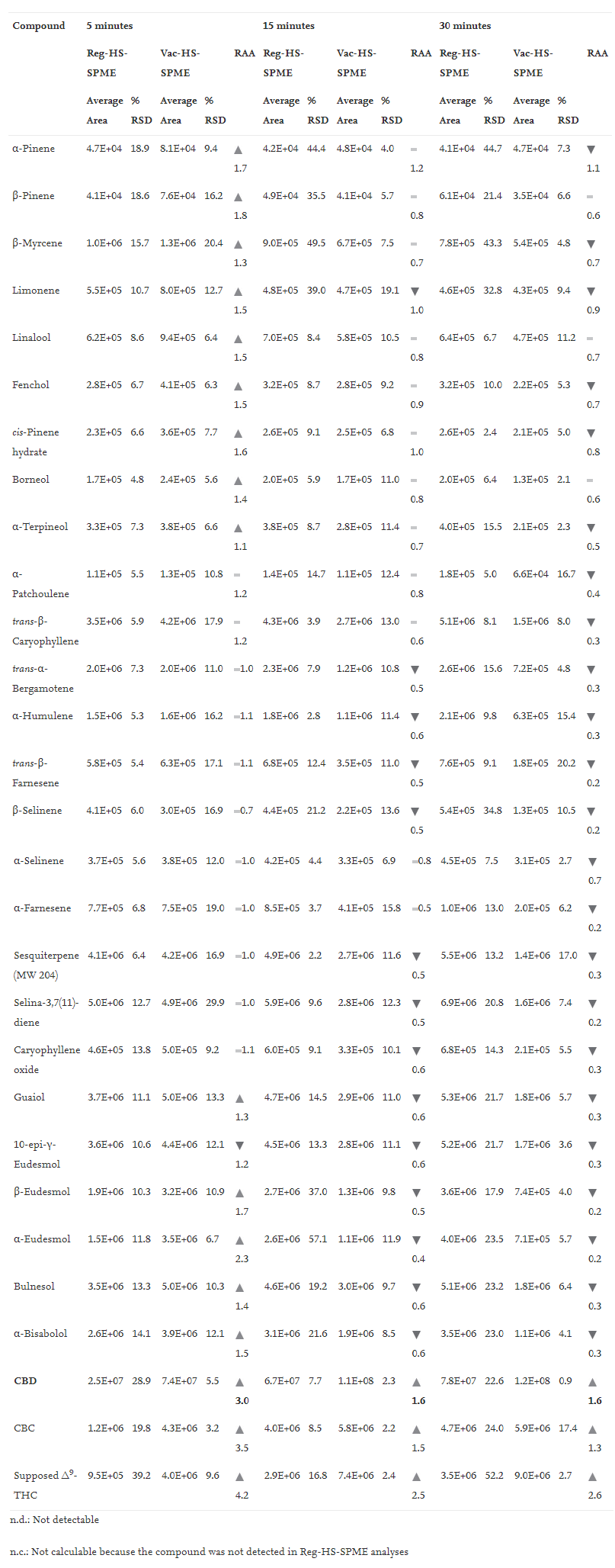
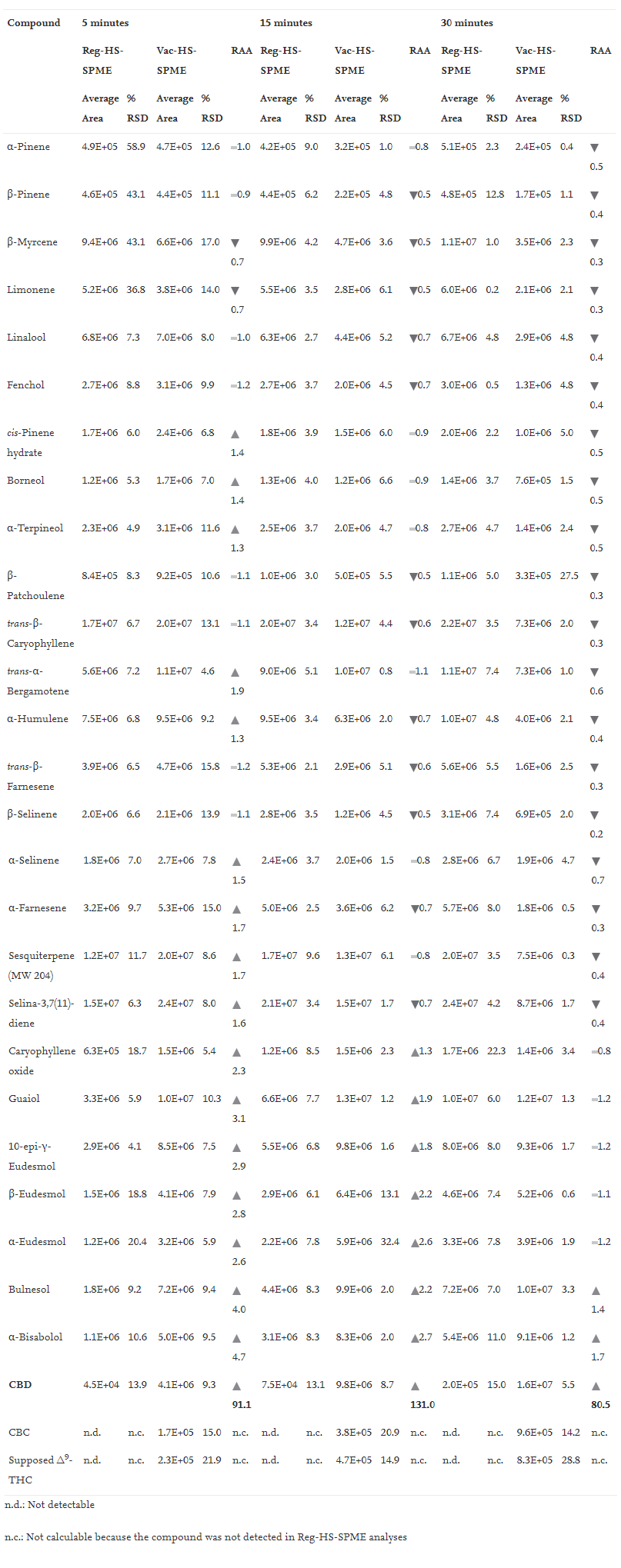
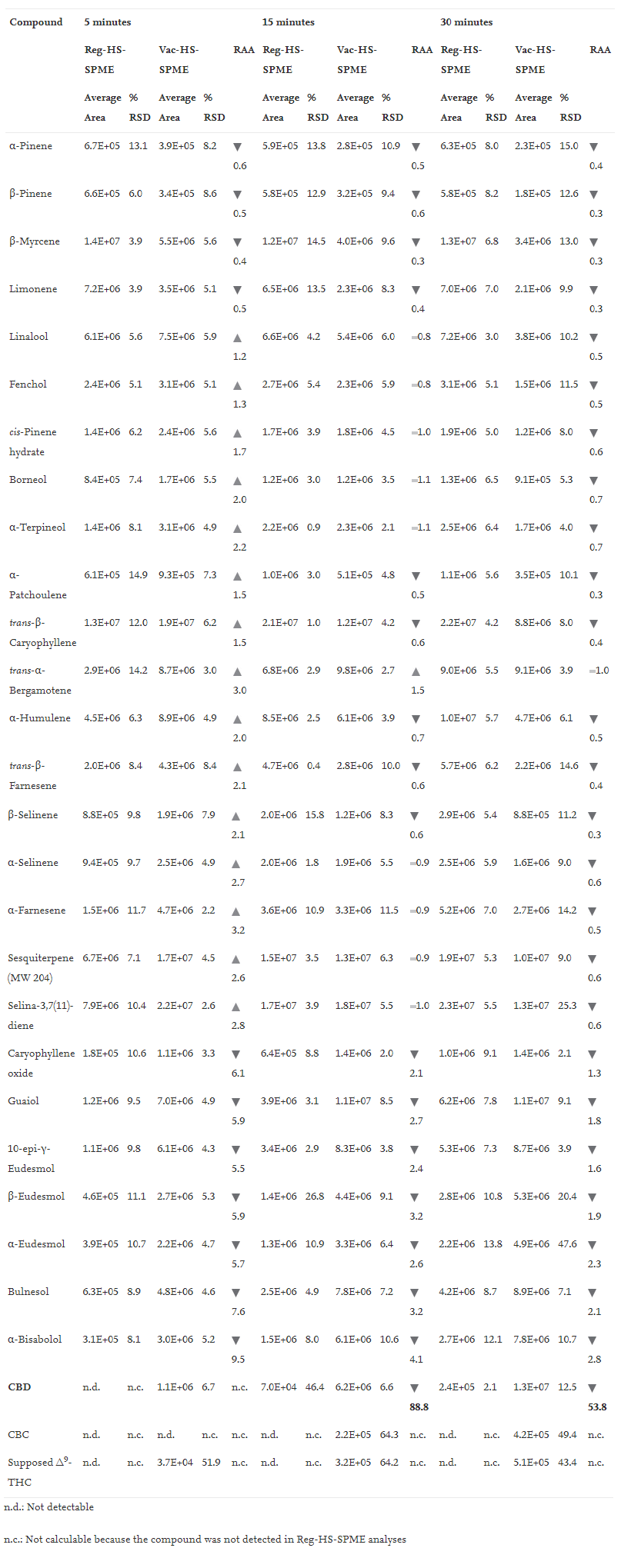
Tables 1, 2, and 3 report (1) the average peak area and % RSD (''n'' = 3) of all the investigated compounds when testing 10 mg of matrix under the different experimental conditions, and (2) the relative analyte abundance (RAA) defined as the ratio between the average peak area obtained under vacuum to that measured at atmospheric pressure conditions at 150°C, 90°C and 80°C, respectively. | |||
[[File:Tab1 Capetti AdvSampPrep2022 2.png|807px]] | |||
{{clear}} | |||
{| | |||
| style="vertical-align:top;" | | |||
{| border="0" cellpadding="5" cellspacing="0" width="807px" | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |<blockquote>'''Table 1''' Mean peak area, %RSD (''n''=3) and Relative Analyte Abundance (RAA) of investigated markers sampled by HSSPME and Vac-HSSPME at 150°C. RAA defines the ratio between Vac-HSSPME and HSSPME area. Legend: red triangle RAA < 0.8; yellow line 0.8 < RAA < 1.2; green triangle RAA > 1.2</blockquote> | |||
|- | |||
|} | |||
|} | |||
[[File:Tab2 Capetti AdvSampPrep2022 2.png|807px]] | |||
{{clear}} | |||
{| | |||
| style="vertical-align:top;" | | |||
{| border="0" cellpadding="5" cellspacing="0" width="807px" | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |<blockquote>'''Table 2''' Mean peak area, %RSD (''n''=3) and Relative Analyte Abundance (RAA) of investigated markers sampled by HSSPME and Vac-HSSPME at 90°C. RAA defines the ratio between Vac-HSSPME and HSSPME area. Legend: red triangle RAA < 0.8; yellow line 0.8 < RAA < 1.2; green triangle RAA > 1.2</blockquote> | |||
|- | |||
|} | |||
|} | |||
[[File:Tab3 Capetti AdvSampPrep2022 2.png|807px]] | |||
{{clear}} | |||
{| | |||
| style="vertical-align:top;" | | |||
{| border="0" cellpadding="5" cellspacing="0" width="807px" | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |<blockquote>'''Table 3''' Mean peak area, %RSD (''n''=3) and Relative Analyte Abundance (RAA) of investigated markers sampled by HSSPME and Vac-HSSPME at 80°C. RAA defines the ratio between Vac-HSSPME and HSSPME area. Legend: red triangle RAA < 0.8; yellow line 0.8 < RAA < 1.2; green triangle RAA > 1.2.</blockquote> | |||
|- | |||
|} | |||
|} | |||
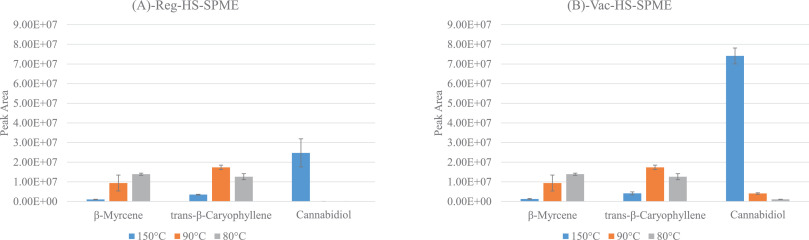
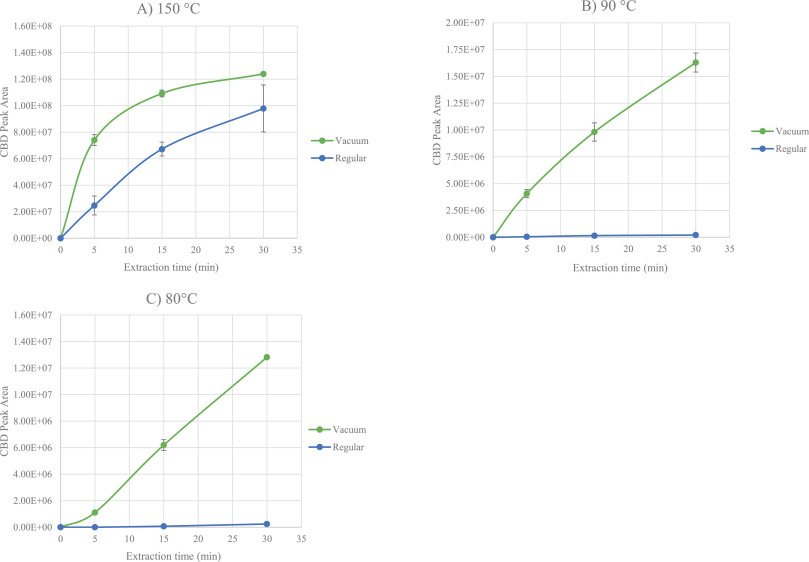
Fig. 2 shows the extraction temperature profiles of β-myrcene, trans-β-caryophyllene, and CBD acquired when sampling 10 mg of the matrix for 5 minutes at the different investigated temperatures. Finally, Fig. 3 provides the extraction times profiles for CBD for each sampling temperature (i.e., 150, 90, and 80°C). In all the cases, the results of both reduced and atmospheric pressure conditions are reported. | |||
[[File:Fig2 Capetti AdvSampPrep2022 2.jpg|809px]] | |||
{{clear}} | |||
{| | |||
| style="vertical-align:top;" | | |||
{| border="0" cellpadding="5" cellspacing="0" width="809px" | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |<blockquote>'''Fig. 2''' Extraction temperature profiles of CBD, β-myrcene, and trans-β-caryophyllene obtained under '''(A)''' HSSPME and '''(B)''' reduced pressure (Vac-HSSPME) conditions. Experimental parameters: PDMS/DVB fiber; 10 mg of sample; 5 minutes of extraction time.</blockquote> | |||
|- | |- | ||
|} | |} | ||
|} | |} | ||
[[File:Fig3 Capetti AdvSampPrep2022 2.jpg|809px]] | |||
{{clear}} | |||
{| | |||
| style="vertical-align:top;" | | |||
{| border="0" cellpadding="5" cellspacing="0" width="809px" | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |<blockquote>'''Fig. 3''' Extraction time profiles for CBD obtained under reduced (Vac-HSSPME; green profile) and regular (HSSPME; blue profile) conditions. Experimental parameters: PDMS/DVB fiber; 10 mg of sample; extraction times 5, 15, 30 minutes; Sampling temperature: (A) 150°C; (B) 90°C; (C) 80°C.</blockquote> | |||
|- | |||
|} | |||
|} | |||
In good agreement with the outcomes of Ilias ''et al.''<ref name=":6" />, sampling under extremely high temperatures (i.e., 150°C) maximized the recovery of CBD even within a short sampling time (i.e., 5 minutes) and under both pressure conditions as stands out in Fig. 2. In addition to CBD, two other less abundant cannabinoids were recovered. One of them is CBC, whose identity was confirmed by comparing its retention time and mass spectrum to those of a certified standard, while the other one is supposed to be Δ<sup>9</sup>-THC, as its mass spectrum matches National Institute of Standards and Technology (NIST) and WILEY MS data, as well as its linear retention index.<ref name=":10" /> Figs. S1, S2 and S4 in the Supplementary materials report the mass spectra of the detected cannabinoids. | |||
The positive effect of vacuum on the recovery of CBD was more important after 5 minutes of sampling (i.e., CBD RAA equal to 3) compared to 15 and 30 minutes (i.e., CBD RAA equal to 1.6), suggesting that equilibrium was being approached after 15 minutes of extraction. In contrast, irrespective of the pressure conditions, sampling at 150°C significantly discriminated against the recovery of the more volatile markers (i.e., mono- and sesquiterpenes). This trend was probably due to the combination of two phenomena: (1) a decrease of the distribution coefficient between the fiber and the headspace (i.e., K<sub>fs</sub>)<ref name=":11" />, and (2) an intensification of competitive adsorption and displacement of low molecular weight analytes given the extremely high amount of extracted CBD.<ref>{{Cite journal |last=Trujillo-Rodríguez |first=María J. |last2=Pino |first2=Verónica |last3=Psillakis |first3=Elefteria |last4=Anderson |first4=Jared L. |last5=Ayala |first5=Juan H. |last6=Yiantzi |first6=Evangelia |last7=Afonso |first7=Ana M. |date=2017-04 |title=Vacuum-assisted headspace-solid phase microextraction for determining volatile free fatty acids and phenols. Investigations on the effect of pressure on competitive adsorption phenomena in a multicomponent system |url=https://linkinghub.elsevier.com/retrieve/pii/S0003267017301472 |journal=Analytica Chimica Acta |language=en |volume=962 |pages=41–51 |doi=10.1016/j.aca.2017.01.056}}</ref> Therefore, 150°C as sampling temperature proved to be inappropriate for the simultaneous characterization of the terpene and cannabinoid profiles. | |||
The recovery of CBD was significantly reduced when sampling at relatively lower temperature (i.e., 80 and 90°C) compared to 150°C. Despite this, very promising results were obtained at 90°C, especially when sampling under reduced pressure conditions. What is striking, as seen in Fig. 3 above, is the steep rise in the extraction kinetic of CBD obtained when sampling at reduced pressure conditions. At 90°C, irrespective of the sampling time, the amount of CBD extracted with vacuum was at least 60 times greater than that recovered under regular conditions, and in just 5 minutes, vacuum ensured the extraction of a sufficient amount of CBD to meet the instrument sensitivity and to provide a good picture of CBD abundance in the inflorescences. As regards the more volatile markers (i.e., β-myrcene and trans-β-caryophyllene), at 90°C their recovery was on average 10 times higher than that obtained at 150°C irrespective of the pressure conditions. As evidenced by the RAA values reported in Table 2 and in Fig. 5 (below), with 5 minutes of sampling, lowering the total pressure in the headspace did not improve the extraction of the monoterpene markers (RAA close to one), while on average it doubled the recovery of sesquiterpenes, as was expected by their lower volatilities. With longer sampling times (i.e., 15 and 30 minutes) the RAA significantly dropped for both mono- and sesquiterpenes, indicating decreased mass loadings under vacuum conditions. | |||
The results obtained when sampling at 80°C were very similar to those observed at 90°C. Under regular conditions, the amount of CBD extracted after 5 minutes was below the instrument limit of detection (signal to noise ratio below three), while when sampling with vacuum the amount of CBD recovered was far above the instrument limit of quantification (signal to noise ratio close to 1000). When sampling at 80°C, the amount of CBD extracted after 5 minutes of sampling was four times lower than that recovered at 90°C, and the repeatability of the extraction of the minor cannabinoids dropped down to RSD values higher than 40%, while there were no significant differences in the recovery of the most volatile markers, irrespective of the extraction times. These results suggest that under vacuum conditions, 90°C is a more suitable extraction temperature compared to 80°C for the simultaneous optimal recovery of both the volatile (i.e., mono- and sesquiterpenes) and semi-volatile markers (i.e., cannabinoids) of ''Cannabis'' inflorescences. | |||
===Thermal stability of CBD under the investigated sampling conditions=== | |||
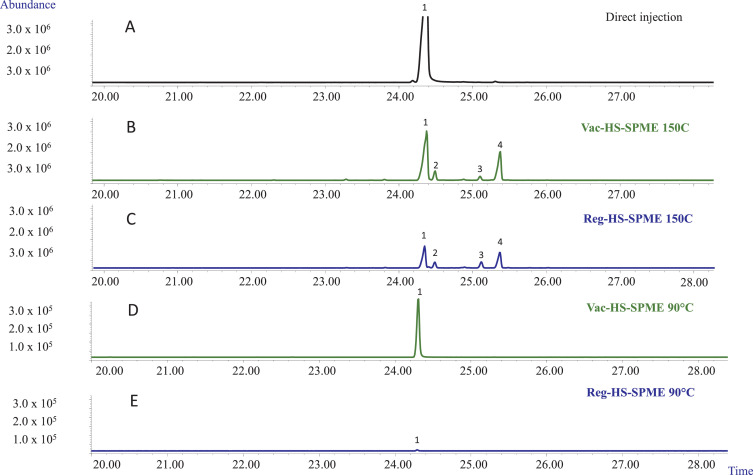
In light of the results obtained by Czégény ''et al.''<ref name=":7" />, the thermal stability of CBD at 150°C and 90°C was studied. First, to ensure that no CDB degradation occurs within the analytical instrument, one µL of a CBD standard solution 1 mg mL<sup>−1</sup> was directly injected and analyzed by GC-MS. Fig. 4A reports the obtained chromatogram, which proved a standard purity in-line with that declared by the manufacturer and excluded the possibility of CBD degradation within the gas chromatograph. | |||
[[File:Fig4 Capetti AdvSampPrep2022 2.jpg|809px]] | |||
{{clear}} | |||
{| | |||
| style="vertical-align:top;" | | |||
{| border="0" cellpadding="5" cellspacing="0" width="809px" | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |<blockquote>'''Fig. 4''' GC-MS profiles of CBD standard solution obtained with MEGA-5 narrow-bore column under the following conditions: '''A''' injection of 1 µL of CBD standard solution 1 mg mL<sup>−1</sup>; '''B''' 10 µL of CBD standard solution 1 mg mL<sup>−1</sup> recovered by Vac-HSSPME after 5 minutes at 150°C; '''C''' 10 µL of CBD standard solution 1 mg mL<sup>−1</sup> recovered by HSSPME after 5 minutes at 150°C; '''D''' 10 µL of CBD standard solution 1 mg mL<sup>−1</sup> recovered after 5 minutes by Vac-HSSPME at 90°C; '''E''' 10 µL of CBD standard solution 1 mg mL<sup>−1</sup> recovered by HSSPME after 5 minutes at 90°C. GC-MS Analysis conditions: see experimental section. Legend: (1) Cannabidiol, (2) Cannabichromene, (3) Cannabinoid 1 (supposed Δ<sup>8</sup>-tetrahydrocannabinol), (4) Cannabinoid 2 (supposed Δ<sup>9</sup>-tetrahydrocannabinol).</blockquote> | |||
|- | |||
|} | |||
|} | |||
[[File:Fig5 Capetti AdvSampPrep2022 2.jpg|809px]] | |||
{{clear}} | |||
{| | |||
| style="vertical-align:top;" | | |||
{| border="0" cellpadding="5" cellspacing="0" width="809px" | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |<blockquote>'''Fig. 5''' HSSPME GC-MS profiles obtained when sampling 10 mg of matrix at 90°C for 5 minutes under reduced (Vac-HSSPME) pressure '''A''' and atmospheric (HSSPME) pressure '''B''' conditions. Legend: (1) α-Pinene, (2) β-Pinene, (3) β-Myrcene, (4) Limonene, (5) Linalool, (6) Fenchol, (7) cis-Pinene hydrate, (8) Borneol, (9) α-Terpineol, (10) β-Patchoulene, (11) trans-β-Caryophyllene, (12) trans-α-Bergamotene, (13) α-Humulene, (14) trans-β-Farnesene (15) β-Selinene, 16) α-Selinene17) α-Farnesene, (18-19) Sesquiterpenes (MW 204), 20) Selina-3,7(11)-diene, (21) trans-Nerolidol, (22) Caryophyllene oxide (23) Guaiol, (24) 10-epi-γ-Eudesmol, (25) β-Eudesmol, (26) α-Eudesmol, (27) Bulnesol, (28) α-Bisabolol, (29) Cannabidiol, (30) Cannabichromene, (31) Cannabinoid 2 (Supposed Δ<sup>9</sup>-THC).</blockquote> | |||
|- | |||
|} | |||
|} | |||
10 µL of the same 1.0 mg mL<sup>−1</sup> CBD standard solution were then sampled by HSSPME, under both pressure conditions for 5 minutes at 150 and 90°C. Three replicates for each sampling temperature were performed. The chromatograms reported in Fig. 4B and C demonstrate that after 5 minutes of extraction at 150°C, under both pressure conditions, CBD undergoes degradation, forming three cannabinoids. The first one is CBC, whose identity was again confirmed by comparing its retention time and mass spectrum to those of a certified standard. The other two cannabinoids are supposed to be Δ<sup>9</sup>-THC and Δ<sup>8</sup>-THC as their mass spectra matched NIST and WILEY MS data, and so did their linear retention index.<ref name=":10" /> The relative amount of CBD undergoing degradation was measured according to the following formula: | |||
:<math>\%{~~}of{~~}degraded{~~}CBD =( \frac{Total{~~}area - Area{~~}CBD}{Total{~~}area}) \times \ 100</math> | |||
where the total area is the sum of the areas of all the detected cannabinoids. The results proved that the percentage of the degraded CBD was not constant amongst the replicates and accounted for 19.5 ± 17.1% and 38.7 ± 17.4% under vacuum and regular conditions, respectively. Cannabinoid 2 (i.e., supposed Δ<sup>9</sup>-THC) was the most abundant degradation products with a relative abundance of 14.6 ± 12.6% and 20.9 ± 15%, under vacuum and regular conditions, respectively. The same degradation trend is not observed by direct injection into the gas chromatograph, even though the CBD is exposed to even higher temperature (i.e., injection port temperature 250°C), probably because the exposure time is too short to cause the degradation. | |||
Fig. 4D and E report the chromatograms obtained when sampling 10 µL of the same 1.0 mg mL<sup>−1</sup> CBD standard solution at 90°C. The results show that, at the investigated temperature, no degradation products were generally detected irrespective of the pressure conditions. Cannabinoid 2 (i.e., supposed Δ<sup>9</sup>-THC) was detected only in one replicate under vacuum condition, but its relative abundance was in any case below 0.6%. These results provide further evidence that relative low sampling temperatures (i.e., 90°C) should be preferred for a more reliable characterization of ''Cannabis'' inflorescences' volatile and semi-volatile fractions. | |||
==Concluding remarks== | |||
The primary aim of this study was to investigate whether Vac-HSSPME could be a suitable sample preparation technique to be combined to fast GC-MS analysis for the simultaneous characterisation of both the volatile (i.e., mono- and sesquiterpenes) and semi-volatile (i.e., cannabinoids) fractions of ''Cannabis sativa'' L. inflorescences. The results proved that compared to standard HSSPME, vacuum conditions in the headspace ensure the fast recovery of cannabinoid markers at considerably lower sampling temperature (i.e., 90°C) that do not discriminate the most volatile fraction nor cause the formation of artifacts when the sampling time is minimized. The possibility of accelerating the subsequent GC-MS analysis, by the use of a short narrow-bore column, was also assessed, and a satisfactory resolution of all the investigated markers was obtained in less than 30 minutes. Overall, since it is fast, totally automatable, and solvent-free, the combination of Vac-HSSPME and fast GC-MS should be considered as a green alternative analytical approach for the characterization of ''Cannabis'' inflorescences. | |||
Despite these very promising results, further experiments are still required to concretize the use of Vac-HSSPME and fast GC-MS analysis for the reliable qualitative and quantitative characterization of ''Cannabis'' samples. These experiments should aim to (1) validate a protocol, based on Vac-HSSPME and fast GC-MS analysis, to quantify specific cannabinoid markers (i.e., CBD and Δ<sup>9</sup>-THC), and (2) prove that the quantification results, obtained at relatively low temperatures, describe the absolute total amount of the investigated cannabinoids (i.e., the sum of the acidic and neutral form in the inflorescence) rather than the only neutral form. | |||
==Supplementary materials== | |||
*[https://ars.els-cdn.com/content/image/1-s2.0-S2772582022000110-mmc1.docx Supplementary Material]: A Microsoft Word document containing tables and figures of supplementary data related to this work. | |||
==Abbreviations, acronyms, and initialisms== | ==Abbreviations, acronyms, and initialisms== | ||
* '''Δ<sup>9</sup>-THC''': Δ<sup>9</sup>-tetrahydrocannabinol | *'''Δ<sup>9</sup>-THC''': Δ<sup>9</sup>-tetrahydrocannabinol | ||
* '''Δ<sup>9</sup>-THCA''': Δ<sup>9</sup>-tetrahydrocannabinolic acid | *'''Δ<sup>9</sup>-THCA''': Δ<sup>9</sup>-tetrahydrocannabinolic acid | ||
* '''CBC''': cannabichromene | *'''CBC''': cannabichromene | ||
* '''CBCA''': cannabichromenic acid | *'''CBCA''': cannabichromenic acid | ||
* '''CBD''': cannabidiol | *'''CBD''': cannabidiol | ||
* '''CBDA''': cannabidiolic acid | *'''CBDA''': cannabidiolic acid | ||
* '''CBEA''': cannabielsoic acid | *'''CBEA''': cannabielsoic acid | ||
* '''CBG''': cannabigerol | *'''CBG''': cannabigerol | ||
* '''CBGA''': cannabigerolic acid | *'''CBGA''': cannabigerolic acid | ||
* '''CBN''': cannabinol | *'''CBN''': cannabinol | ||
* '''CBNA''': cannabinolic acid | *'''CBNA''': cannabinolic acid | ||
* '''CBNDA''': cannabinodiolic acid | *'''CBNDA''': cannabinodiolic acid | ||
* '''GC''': gas chromatography | *'''GC''': gas chromatography | ||
* '''GC-FID-MS''': gas chromatography–flame ionization detector–mass spectrometry | *'''GC-FID-MS''': gas chromatography–flame ionization detector–mass spectrometry | ||
* '''GC-MS''': Gas chromatography–mass spectrometry | *'''GC-MS''': Gas chromatography–mass spectrometry | ||
* '''HPLC''': high-performance liquid chromatography | *'''HPLC''': high-performance liquid chromatography | ||
* '''HSSPME''': headspace solid-phase microextraction | *'''HSSPME''': headspace solid-phase microextraction | ||
* '''SLE''': solid-liquid extraction | *'''SLE''': solid-liquid extraction | ||
* '''SPME''': solid-phase microextraction | *'''SPME''': solid-phase microextraction | ||
* '''Vac-HSSPME''': vacuum-assisted headspace solid-phase microextraction | *'''Vac-HSSPME''': vacuum-assisted headspace solid-phase microextraction | ||
==Acknowledgements== | |||
The authors are indebted to Professor Eleftheria Psillakis, who provided helpful consultancy and advice. This article is based upon work from the Sample Preparation Study Group and Network, supported by the Division of Analytical Chemistry of the European Chemical Society. | |||
===Funding=== | |||
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. | |||
===Competing interests=== | |||
The authors declare no conflict of interest. | |||
==References== | ==References== | ||
| Line 133: | Line 260: | ||
<!--Place all category tags here--> | <!--Place all category tags here--> | ||
[[Category: | [[Category:LIMSwiki journal articles (added in 2022)]] | ||
[[Category: | [[Category:LIMSwiki journal articles (all)]] | ||
[[Category: | [[Category:LIMSwiki journal articles on cannabis cannabinoids]] | ||
[[Category: | [[Category:LIMSwiki journal articles on cannabis terpenes]] | ||
[[Category: | [[Category:LIMSwiki journal articles on cannabis testing]] | ||
[[Category:LIMSwiki journal articles (with rendered math)]] | |||
Latest revision as of 23:59, 26 December 2023
| Full article title | A sustainable approach for the reliable and simultaneous determination of terpenoids and cannabinoids in hemp inflorescences by vacuum-assisted headspace solid-phase microextraction |
|---|---|
| Journal | Advances in Sample Preparation |
| Author(s) | Capetti, Francesca; Rubiolo, Patrizia; Mastellone, Giulia; Marengo, Arianna; Sgorbini, Barbara; Cagliero, Cecilia |
| Author affiliation(s) | Università di Torino |
| Primary contact | Email: cecilia dot cagliero at unito dot it |
| Year published | 2022 |
| Volume and issue | 2 |
| Article # | 100014 |
| DOI | 10.1016/j.sampre.2022.100014 |
| ISSN | 2772-5820 |
| Distribution license | Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International |
| Website | https://www.sciencedirect.com/science/article/pii/S2772582022000110 |
| Download | https://www.sciencedirect.com/science/article/pii/S2772582022000110/pdfft (PDF) |
|
|
This article contains rendered mathematical formulae. You may require the TeX All the Things plugin for Chrome or the Native MathML add-on and fonts for Firefox if they don't render properly for you. |
Abstract
Cannabis sativa L. is an intriguing plant that has been exploited since ancient times for recreational, medical, textile and food purposes. The plant's most promising bioactive constituents discovered so far belong to the terpenoid and cannabinoid classes. These specialized metabolites are highly concentrated in the plant aerial parts, and their chemical characterization is crucial to guarantee the safe and efficient use of the plant material irrespective of which use it is.
This study investigates for the first time the use of vacuum-assisted headspace solid-phase microextraction (Vac-HSSPME) as a sample preparation process in an analytical protocol based on Vac-HSSPME combined to fast gas chromatography–mass spectrometry (GC-MS) analysis that aims at comprehensively characterizing both the terpenoid and cannabinoid profiles of Cannabis inflorescences in a single step. The results proved that vacuum in the headspace should be preferred over atmospheric pressure conditions as it ensures the fast recovery of cannabinoid markers at relatively lower sampling temperatures (i.e., 90°C) that do not discriminate the most volatile fraction nor cause the formation of artifacts when the sampling time is minimized.
Keywords: Cannabis sativa inflorescences, vacuum-assisted headspace solid-phase microextraction, volatilome, terpenoids, cannabinoids
Introduction
Cannabis sativa L. currently can be considered one of the most studied plants due to its relevance in the illicit drug market and in the textile and food industry[1], as well as its potential medical usage. Whether the plant is intended for recreational purposes, fiber production (hemp), or medical use depends on the content of two major cannabinoids in the aerial parts of the plant: the psychoactive (-)-trans-Δ9-tetrahydrocannabinol (Δ9-THC) and cannabidiol (CBD), the latter displaying several biological activities but not the psychotropic one.[2][3] The illicit drug chemotype, also known as type I, contains an excess of Δ9-THC and a limited amount of CBD. Contrary to that is the cannabis chemotype used in manufacturing (i.e., industrial hemp or type III), where the ratio is reversed and Δ9-THC content cannot exceed 0.2%. Finally, the type II chemotype, which is used for medical purposes, is defined as having high mean contents of both CBD and Δ9-THC (i.e., Bedrocan: 22% THC, <1% CBD; Bediol: 6.5% THC, 8% CBD).[3][4][5]
Other than Δ9-THC and CBD, the plant may synthesize several specialised metabolites, including additional phytocannabinoids and terpenes, amongst others[6], which are both produced by stalked glandular trichomes that are highly concentrated on female inflorescences.[7] Phytocannabinoids are C21 compounds known as terpenophenolic compounds. They are produced by the plant in their acidic form, which under heating or during storage is decarboxylated into the active neutral form.[6] At least 104 cannabinoids have been isolated so far.[8] Aside from Δ9-THC, CBD, cannabichromene (CBC), and cannabigerol (CBG), other predominant secondary metabolites are Δ9-tetrahydrocannabinolic acid (Δ9-THCA), cannabidiolic acid (CBDA), cannabichromenic acid (CBCA), and cannabigerolic acid (CBGA), which are the precursors of the formerly mentioned compounds. Other minor cannabinoids include cannabinolic acid (CBNA) and Δ8THCA, which are artifacts of Δ9THCA, as well as cannabielsoic acid (CBEA) and cannabinodiolic acid (CBNDA), which derive from CBDA.[9]
Terpenes are cannabis's most abundant specialized metabolites, including at least 120 identified terpenoids.[8] Literature data suggest that varying pharmaceutical properties between different Cannabis varieties can be attributed to synergistic interactions, known as the "entourage effect," between cannabinoids and terpenes.[9] A comprehensive qualitative characterization of both the cannabinoid and terpene profiles of the plant raw material is therefore of utmost importance not only to define its rational use (i.e., whether the plant under investigation was cultivated for fiber production, medical, or drug purposes) but also to guarantee the efficacy and safety of its potential pharmaceutical application.
The most employed method of extraction of cannabinoids from plant raw material is solid-liquid extraction (SLE) using ethanol or acetone as extracting solvents due to their affinity and consequent high extracting efficiency for cannabinoids.[10][11] High-performance liquid chromatography (HPLC) and gas chromatography coupled to mass spectrometry (GC-MS) are the analytical techniques of choice for many qualitative and quantitative analyses.[11] HPLC is usually employed when the acid and the neutral form of the investigated cannabinoids must be measured separately, while GC analyses enable the characterization of the total cannabinoid content (e.g. the combined amount of THC and THCA), as GC systems, by definition, work with high temperatures that lead unavoidably to the decarboxylation of cannabinoid acids.[11] The total cannabinoid content is usually measured as it best represents the pharmacological activity of the material, unless differently stated by legislation.[12]
Thanks to their volatile nature, the isolation of terpenes—and in particular of mono- and sesquiterpenes—from plant raw material is straightforward, and their profiling can be performed by headspace solid-phase microextraction (HSSPME) online combined to GC-MS analysis.[5]
The recovery of cannabinoids from solid matrices by HSSPME is also feasible but requires long sampling times in combination to high sampling temperatures due to their low volatility and low tendency to escape to the headspace. In 2004, Lachenmeier et al. optimized a successful HSSPME method followed by on-coating derivatization of the cannabinoids with N-methyl-N-trimethylsilyltrifluoroacetamide (MSTFA) for the extraction of cannabinoids from hemp food products using 90°C as sampling temperature and 30 minutes of extraction time[13], while in 2005, Ilias et al. showed that cannabinoid extraction should be performed at 150°C to maximize their recovery in short sampling times (e.g., five minutes).[14] However, in more recent work, Czégény et al. investigated the effect of temperature on the composition of pyrolysis products of CBD in e-cigarettes. They tested different operating temperatures (250–400°C), and they proved that, depending on the temperature and atmosphere (i.e., inert of oxidative condition), 25–52% of CBD can be converted into other cannabinoids, amongst which Δ9-THC, Δ8-THC, cannabinol (CBN), and CBC are the predominant pyrolysates.[15] Even though when performing HSSPME it is usually unlikely to reach such extreme temperatures, the results of Czégény et al. suggest that (1) reduced sampling temperature should be preferred to obtain a truthful cannabinoid fingerprint profile in the plant raw material, and (2) CBD potential degradation should be investigated during the optimization of the sampling temperature.
As thoroughly described by Psillakis et al.[16][17][18][19], vacuum is a powerful experimental parameter to consider for increasing the extraction kinetic of semi-volatile compounds during the HSSPME process. This is because in the case of semi-volatiles and under non-equilibrium conditions, a reduced pressure inside the sample container decreases the resistance to mass transfer in the gas zone at the solid-headspace interface. As a consequence, higher extraction efficiencies for semi-volatile compounds can be achieved in shorter sampling times, and potentially at milder extraction temperatures.[20][21]
This study investigates the advantages and disadvantages of using vacuum-assisted headspace solid-phase microextraction (Vac-HSSPME) over HSSPME as a sample preparation process to be exploited in analytical protocols, aiming to comprehensively characterize both the terpene and cannabinoid profiles of Cannabis inflorescences in a single step while employing a total analysis system.
Materials and methods
Chemicals and samples
CBD and CBC standard solutions 1.0 mg mL−1 in methanol were purchased from Merck KGaA, Darmstadt, Germany. Dried Cannabis inflorescences of type III chemotype were purchased from an authorized local hemp shop. The dried plant material was pulverized by an electric blender and stored at -18°C.
Procedures under reduced (Vac-HSSPME) and atmospheric pressure conditions (HSSPME)
Divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) 50/30 μm (2 cm length) and over coated PDMS/DVB 75 μm (coating thickness includes 65 μm coating + 10 μm OC [overcoating]) fibers were employed for the experiments. The fibers were purchased from Merck KGaA, Darmstadt, Germany and conditioned following the manufacturer's instructions.
HSSPME experiments were performed using conventional headspace 20 mL crimp vials provided by Restek, Bellefonte, USA, as well as a 20 mm magnetic ring crimp cap, fitted with 20.6 mm septa (Buty red/PTFE grey, 55 shore A, 1.3 mm).
Vac-HSSPME experiments were performed in the same commercial headspace 20 mL crimp vials but hermetically sealed with a stainless steel closure (provided by Prof. Elefteria Psillakis) having a hole that could tightly accommodate a Thermogreen LB-1 septum with half-hole (Supelco, Bellefonte, USA), through which the air evacuation step and the SPME sampling were performed.
For the inflorescences, 10 mg of the pulverized sample were placed inside the vial, which was again stored at −18°C for one hour and then air-evacuated.[20] The air-evacuation step was performed with a 22-gauge hypodermic needle sealed to a 5 mL syringe tightly secured to the tubing of a N 820.3 FT.18 (7 mbar ultimate vacuum) pumping unit manufactured by KNF Lab (Milan, Italy). The needle was inserted through the septum, and the vial was air-evacuated for one minute. The procedure was the same for HSSPME, while omitting the air evacuation step.
For CBD standard sampling, 10 µL of the 1.0 mg mL−1 solution were introduced through the closure septum after the air-evacuation step. For HSSPME experiments, the liquid sample was introduced into the vial by the open-vial technique.[22]
After sampling, the fiber was withdrawn and the SPME device moved to the GC-MS system for analysis. GC desorption lasted 10 minutes to minimize carry-over.
Ten milligrams of pulverized plant material were sampled at three different extraction temperatures (i.e., 80, 90 and 150°C) under both Vac-HSSPME and HSSPME experiments. Sampling-time profiles were obtained for all the above mentioned conditions by sampling for 5, 15, and 30 minutes. 10 µL of CBD standard solution 1.0 mg mL−1 were sampled at 90 and 150°C for 5 minutes, under both pressure conditions. All extractions were run in triplicate.
Instrument set-up
GC-MS systems and columns
Analyses were carried out on two different instruments: (1) a MPS-2 multipurpose sampler (Gerstel, Mülheim a/d Ruhr, Germany) installed on a Shimadzu gas chromatography–flame ionization detector–mass spectrometry (GC-FID-MS) system, consisting of a Shimadzu GC 2010 system, equipped with FID, in parallel with a Shimadzu QP2010-PLUS GC–MS mass spectrometer (Shimadzu, Milan, Italy); (2) a MPS-2 multipurpose sampler (Gerstel, Mülheim a/d Ruhr, Germany) installed on an Agilent 6890 N GC system coupled to a 5975 MSD mass spectrometer (Agilent Technologies, Santa Clara, CA).
GC analyses were carried out using two MEGA-5 95% methyl-polysiloxane 5%-phenyl (MEGA, Legnano, MI, Italy) columns: a conventional 30 m × 0.25 mm dc, 0.25 µm df column installed on the Agilent 6890 N GC - 5975 MSD, and a narrow-bore 15 m × 0.18 mm dc, 0.18 µm df column installed on the Shimadzu GCMS-QP2010.
Data was processed with the ChemStation Version E.02.02.1431 data processing system (Agilent Technologies, Santa Clara, CA).
GC-MS conditions
Analyses were carried out under the following conditions. Temperatures: injector: 250 °C, transfer line: 270 °C, ion source: 200 °C; carrier gas: He; flow control mode: constant linear velocity; flow rate: 1.00 mL min−1 (conventional column), 0.72 mL min−1 (narrow bore column); injection mode: split; split ratio: 1:20.
The MS was operated in electron ionization mode (EI) at 70 eV, scan rate: 666 u/s, mass range: 35–350 m/z. Temperature programs: (i) 50°C (one minute)// 3°C/min//250°C (five minutes) for conventional MEGA-%; (ii) 50°C (30 seconds) //7.2°C/min// 250°C (two minutes) for the narrow-bore column. The chromatographic conditions for the narrow-bore columns were obtained by translating the method parameters through the Agilent method translator software.[23] Identification was performed via comparisons of linear retention indices and mass spectra either with those of authentic standards, or with data stored in commercial[24] and in-house libraries.
Results and discussion
Table S1 in the Supplementary materials provides the list of the target compounds together with their physicochemical properties (i.e., LogKow, boiling point, and vapor pressure). The mono- and sesquiterpene markers to be investigated were chosen according to the results of Jin et al., who comprehensively profiled reference specialized metabolites in Cannabis inflorescences, leaves, stem barks, and roots for the three Cannabis chemotypes (i.e., Type I, II and III).[8]
Preliminary optimization of the fiber coating and chromatographic conditions
The first set of experiments aimed at selecting (1) the optimum fiber coating that could extract all the investigated analytes with acceptable sensitivity and (2) the best chromatographic conditions providing an acceptable resolution of all the investigated markers in a reasonable time for high-throughput analyses. In our study, two fiber coatings were tested: the PDMS/DVB and the DVB/CAR/PDMS fibers. Fig. 1A and B show the profiles obtained with the two investigated coatings when sampling 10 mg of matrix with HSSPME at 90°C for 30 minutes of extraction. The chromatographic analyses were performed employing a conventional 30 m × 0.25 mm dc, 0.25 µm df MEGA-5 column. Irrespective of the fiber coating, the most abundant compounds amongst the recovered mono- and sesquiterpenes were β-myrcene (3), trans-β-caryophyllene (11), and selina-3,7(11)-diene (20), while only one cannabinoid—CBD (29)—was recovered. In agreement with the results of Ilias et al.[14], the PDMS/DVB fiber proved to be more efficient for the recovery of CBD compared to the triphasic fiber which, however, extracted a quantitative richer mono- and sesquiterpene profile. The PDMS/DVB fiber was chosen for the subsequent experiments because of its higher recovery of CBD while still providing an acceptable sensitivity for the investigated mono- and sesquiterpene metabolites.
The employed conventional MEGA-5 column proved to separate the main selected markers with an acceptable resolution. The possibility to improve the analysis speed and to decrease the use of gas with a greener analytical protocol was then investigated. The chromatographic analysis was sped up by translating the method to a 15 m × 0.18 mm dc, 0.18 µm df column using the method translation approach.[23][25][26] Fig. 1C reports the translated GC-MS profile of the investigated hemp inflorescences with the narrow-bore MEGA-5 column. The analysis time was reduced from 72.67 to 30.28 minutes, while maintaining the separation and the elution pattern of the investigated markers.
|
Vac-HSSPME and HSSPME
Due to their relative high molecular weights and boiling points (e.g., CBD 428.52°C, 760 mmHg[27]) cannabinoids have a low tendency to escape to headspace and require high sampling temperatures to be recovered by HSSPME.[14] However, in view of a reliable and comprehensive HSSPME method suitable for the simultaneous qualitative characterization of both the terpenoid and the cannabinoid fractions of Cannabis inflorescences, high sampling temperatures are not advisable for two reasons. First, they may decrease the distribution coefficient between the fiber and the headspace (i.e., Kfs) of the most volatile components, reducing their recovery.[28] In addition, a high sampling temperature, especially when combined with relatively long extraction times, may induce decomposition of some compounds and/or creation of other components or artifacts.[29] According to the theory, a reduced pressure inside the headspace sample container increases the compounds' molecular diffusion coefficient in air and favors their vapor flux at the solid surface. As a result, reducing the total headspace pressure is an alternative and complementary strategy, compared to the adoption of high sampling temperatures, to speed up the extraction kinetic of semi-volatile compounds.[30]
In this study we compared the performances of Vac-HSSPME to those of HSSPME under different sampling temperatures (i.e., 150°C, 90°C, and 80°C) and extraction times (i.e., 5, 15, 30 minutes), and we explored whether Vac-HSSPME could be a more suitable sample preparation technique for the simultaneous characterization of both the terpenoid and cannabinoid profiles of Cannabis inflorescences. For the following discussion, CBD will be considered as representative of the plant cannabinoids, while β-myrcene and trans-β-caryophyllene will be considered representative of the mono- and sesquiterpene markers, respectively.
Tables 1, 2, and 3 report (1) the average peak area and % RSD (n = 3) of all the investigated compounds when testing 10 mg of matrix under the different experimental conditions, and (2) the relative analyte abundance (RAA) defined as the ratio between the average peak area obtained under vacuum to that measured at atmospheric pressure conditions at 150°C, 90°C and 80°C, respectively.
|
|
|
Fig. 2 shows the extraction temperature profiles of β-myrcene, trans-β-caryophyllene, and CBD acquired when sampling 10 mg of the matrix for 5 minutes at the different investigated temperatures. Finally, Fig. 3 provides the extraction times profiles for CBD for each sampling temperature (i.e., 150, 90, and 80°C). In all the cases, the results of both reduced and atmospheric pressure conditions are reported.
|
|
In good agreement with the outcomes of Ilias et al.[14], sampling under extremely high temperatures (i.e., 150°C) maximized the recovery of CBD even within a short sampling time (i.e., 5 minutes) and under both pressure conditions as stands out in Fig. 2. In addition to CBD, two other less abundant cannabinoids were recovered. One of them is CBC, whose identity was confirmed by comparing its retention time and mass spectrum to those of a certified standard, while the other one is supposed to be Δ9-THC, as its mass spectrum matches National Institute of Standards and Technology (NIST) and WILEY MS data, as well as its linear retention index.[24] Figs. S1, S2 and S4 in the Supplementary materials report the mass spectra of the detected cannabinoids.
The positive effect of vacuum on the recovery of CBD was more important after 5 minutes of sampling (i.e., CBD RAA equal to 3) compared to 15 and 30 minutes (i.e., CBD RAA equal to 1.6), suggesting that equilibrium was being approached after 15 minutes of extraction. In contrast, irrespective of the pressure conditions, sampling at 150°C significantly discriminated against the recovery of the more volatile markers (i.e., mono- and sesquiterpenes). This trend was probably due to the combination of two phenomena: (1) a decrease of the distribution coefficient between the fiber and the headspace (i.e., Kfs)[28], and (2) an intensification of competitive adsorption and displacement of low molecular weight analytes given the extremely high amount of extracted CBD.[31] Therefore, 150°C as sampling temperature proved to be inappropriate for the simultaneous characterization of the terpene and cannabinoid profiles.
The recovery of CBD was significantly reduced when sampling at relatively lower temperature (i.e., 80 and 90°C) compared to 150°C. Despite this, very promising results were obtained at 90°C, especially when sampling under reduced pressure conditions. What is striking, as seen in Fig. 3 above, is the steep rise in the extraction kinetic of CBD obtained when sampling at reduced pressure conditions. At 90°C, irrespective of the sampling time, the amount of CBD extracted with vacuum was at least 60 times greater than that recovered under regular conditions, and in just 5 minutes, vacuum ensured the extraction of a sufficient amount of CBD to meet the instrument sensitivity and to provide a good picture of CBD abundance in the inflorescences. As regards the more volatile markers (i.e., β-myrcene and trans-β-caryophyllene), at 90°C their recovery was on average 10 times higher than that obtained at 150°C irrespective of the pressure conditions. As evidenced by the RAA values reported in Table 2 and in Fig. 5 (below), with 5 minutes of sampling, lowering the total pressure in the headspace did not improve the extraction of the monoterpene markers (RAA close to one), while on average it doubled the recovery of sesquiterpenes, as was expected by their lower volatilities. With longer sampling times (i.e., 15 and 30 minutes) the RAA significantly dropped for both mono- and sesquiterpenes, indicating decreased mass loadings under vacuum conditions.
The results obtained when sampling at 80°C were very similar to those observed at 90°C. Under regular conditions, the amount of CBD extracted after 5 minutes was below the instrument limit of detection (signal to noise ratio below three), while when sampling with vacuum the amount of CBD recovered was far above the instrument limit of quantification (signal to noise ratio close to 1000). When sampling at 80°C, the amount of CBD extracted after 5 minutes of sampling was four times lower than that recovered at 90°C, and the repeatability of the extraction of the minor cannabinoids dropped down to RSD values higher than 40%, while there were no significant differences in the recovery of the most volatile markers, irrespective of the extraction times. These results suggest that under vacuum conditions, 90°C is a more suitable extraction temperature compared to 80°C for the simultaneous optimal recovery of both the volatile (i.e., mono- and sesquiterpenes) and semi-volatile markers (i.e., cannabinoids) of Cannabis inflorescences.
Thermal stability of CBD under the investigated sampling conditions
In light of the results obtained by Czégény et al.[15], the thermal stability of CBD at 150°C and 90°C was studied. First, to ensure that no CDB degradation occurs within the analytical instrument, one µL of a CBD standard solution 1 mg mL−1 was directly injected and analyzed by GC-MS. Fig. 4A reports the obtained chromatogram, which proved a standard purity in-line with that declared by the manufacturer and excluded the possibility of CBD degradation within the gas chromatograph.
|
|
10 µL of the same 1.0 mg mL−1 CBD standard solution were then sampled by HSSPME, under both pressure conditions for 5 minutes at 150 and 90°C. Three replicates for each sampling temperature were performed. The chromatograms reported in Fig. 4B and C demonstrate that after 5 minutes of extraction at 150°C, under both pressure conditions, CBD undergoes degradation, forming three cannabinoids. The first one is CBC, whose identity was again confirmed by comparing its retention time and mass spectrum to those of a certified standard. The other two cannabinoids are supposed to be Δ9-THC and Δ8-THC as their mass spectra matched NIST and WILEY MS data, and so did their linear retention index.[24] The relative amount of CBD undergoing degradation was measured according to the following formula:
where the total area is the sum of the areas of all the detected cannabinoids. The results proved that the percentage of the degraded CBD was not constant amongst the replicates and accounted for 19.5 ± 17.1% and 38.7 ± 17.4% under vacuum and regular conditions, respectively. Cannabinoid 2 (i.e., supposed Δ9-THC) was the most abundant degradation products with a relative abundance of 14.6 ± 12.6% and 20.9 ± 15%, under vacuum and regular conditions, respectively. The same degradation trend is not observed by direct injection into the gas chromatograph, even though the CBD is exposed to even higher temperature (i.e., injection port temperature 250°C), probably because the exposure time is too short to cause the degradation.
Fig. 4D and E report the chromatograms obtained when sampling 10 µL of the same 1.0 mg mL−1 CBD standard solution at 90°C. The results show that, at the investigated temperature, no degradation products were generally detected irrespective of the pressure conditions. Cannabinoid 2 (i.e., supposed Δ9-THC) was detected only in one replicate under vacuum condition, but its relative abundance was in any case below 0.6%. These results provide further evidence that relative low sampling temperatures (i.e., 90°C) should be preferred for a more reliable characterization of Cannabis inflorescences' volatile and semi-volatile fractions.
Concluding remarks
The primary aim of this study was to investigate whether Vac-HSSPME could be a suitable sample preparation technique to be combined to fast GC-MS analysis for the simultaneous characterisation of both the volatile (i.e., mono- and sesquiterpenes) and semi-volatile (i.e., cannabinoids) fractions of Cannabis sativa L. inflorescences. The results proved that compared to standard HSSPME, vacuum conditions in the headspace ensure the fast recovery of cannabinoid markers at considerably lower sampling temperature (i.e., 90°C) that do not discriminate the most volatile fraction nor cause the formation of artifacts when the sampling time is minimized. The possibility of accelerating the subsequent GC-MS analysis, by the use of a short narrow-bore column, was also assessed, and a satisfactory resolution of all the investigated markers was obtained in less than 30 minutes. Overall, since it is fast, totally automatable, and solvent-free, the combination of Vac-HSSPME and fast GC-MS should be considered as a green alternative analytical approach for the characterization of Cannabis inflorescences.
Despite these very promising results, further experiments are still required to concretize the use of Vac-HSSPME and fast GC-MS analysis for the reliable qualitative and quantitative characterization of Cannabis samples. These experiments should aim to (1) validate a protocol, based on Vac-HSSPME and fast GC-MS analysis, to quantify specific cannabinoid markers (i.e., CBD and Δ9-THC), and (2) prove that the quantification results, obtained at relatively low temperatures, describe the absolute total amount of the investigated cannabinoids (i.e., the sum of the acidic and neutral form in the inflorescence) rather than the only neutral form.
Supplementary materials
- Supplementary Material: A Microsoft Word document containing tables and figures of supplementary data related to this work.
Abbreviations, acronyms, and initialisms
- Δ9-THC: Δ9-tetrahydrocannabinol
- Δ9-THCA: Δ9-tetrahydrocannabinolic acid
- CBC: cannabichromene
- CBCA: cannabichromenic acid
- CBD: cannabidiol
- CBDA: cannabidiolic acid
- CBEA: cannabielsoic acid
- CBG: cannabigerol
- CBGA: cannabigerolic acid
- CBN: cannabinol
- CBNA: cannabinolic acid
- CBNDA: cannabinodiolic acid
- GC: gas chromatography
- GC-FID-MS: gas chromatography–flame ionization detector–mass spectrometry
- GC-MS: Gas chromatography–mass spectrometry
- HPLC: high-performance liquid chromatography
- HSSPME: headspace solid-phase microextraction
- SLE: solid-liquid extraction
- SPME: solid-phase microextraction
- Vac-HSSPME: vacuum-assisted headspace solid-phase microextraction
Acknowledgements
The authors are indebted to Professor Eleftheria Psillakis, who provided helpful consultancy and advice. This article is based upon work from the Sample Preparation Study Group and Network, supported by the Division of Analytical Chemistry of the European Chemical Society.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interests
The authors declare no conflict of interest.
References
- ↑ Pellati, Federica; Brighenti, Virginia; Sperlea, Johanna; Marchetti, Lucia; Bertelli, Davide; Benvenuti, Stefania (14 October 2018). "New Methods for the Comprehensive Analysis of Bioactive Compounds in Cannabis sativa L. (hemp)" (in en). Molecules 23 (10): 2639. doi:10.3390/molecules23102639. ISSN 1420-3049. PMC PMC6222702. PMID 30322208. http://www.mdpi.com/1420-3049/23/10/2639.
- ↑ Appendino, G.; Chianese, G.; Taglialatela-Scafati, O. (1 March 2011). "Cannabinoids: Occurrence and Medicinal Chemistry" (in en). Current Medicinal Chemistry 18 (7): 1085–1099. doi:10.2174/092986711794940888. http://www.eurekaselect.com/openurl/content.php?genre=article&issn=0929-8673&volume=18&issue=7&spage=1085.
- ↑ 3.0 3.1 Jin, Dan; Henry, Philippe; Shan, Jacqueline; Chen, Jie (1 July 2021). "Identification of Chemotypic Markers in Three Chemotype Categories of Cannabis Using Secondary Metabolites Profiled in Inflorescences, Leaves, Stem Bark, and Roots". Frontiers in Plant Science 12: 699530. doi:10.3389/fpls.2021.699530. ISSN 1664-462X. PMC PMC8283674. PMID 34276749. https://www.frontiersin.org/articles/10.3389/fpls.2021.699530/full.
- ↑ European Monitoring Centre for Drugs and Drug Addiction (2018). Cannabis legislation in Europe: An overview. LU: Publications Office. pp. 1–30. doi:10.2810/930744. https://data.europa.eu/doi/10.2810/930744.
- ↑ 5.0 5.1 Calvi, Lorenzo; Pentimalli, Daniela; Panseri, Sara; Giupponi, Luca; Gelmini, Fabrizio; Beretta, Giangiacomo; Vitali, Davide; Bruno, Massimo et al. (1 February 2018). "Comprehensive quality evaluation of medical Cannabis sativa L. inflorescence and macerated oils based on HS-SPME coupled to GC–MS and LC-HRMS (q-exactive orbitrap®) approach" (in en). Journal of Pharmaceutical and Biomedical Analysis 150: 208–219. doi:10.1016/j.jpba.2017.11.073. https://linkinghub.elsevier.com/retrieve/pii/S0731708517325086.
- ↑ 6.0 6.1 Andre, Christelle M.; Hausman, Jean-Francois; Guerriero, Gea (4 February 2016). "Cannabis sativa: The Plant of the Thousand and One Molecules". Frontiers in Plant Science 7. doi:10.3389/fpls.2016.00019. ISSN 1664-462X. PMC PMC4740396. PMID 26870049. http://journal.frontiersin.org/Article/10.3389/fpls.2016.00019/abstract.
- ↑ Russo, Ethan B (1 August 2011). "Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects: Phytocannabinoid-terpenoid entourage effects" (in en). British Journal of Pharmacology 163 (7): 1344–1364. doi:10.1111/j.1476-5381.2011.01238.x. PMC PMC3165946. PMID 21749363. https://onlinelibrary.wiley.com/doi/10.1111/j.1476-5381.2011.01238.x.
- ↑ 8.0 8.1 8.2 Jin, Dan; Dai, Kaiping; Xie, Zhen; Chen, Jie (1 December 2020). "Secondary Metabolites Profiled in Cannabis Inflorescences, Leaves, Stem Barks, and Roots for Medicinal Purposes" (in en). Scientific Reports 10 (1): 3309. doi:10.1038/s41598-020-60172-6. ISSN 2045-2322. PMC PMC7039888. PMID 32094454. http://www.nature.com/articles/s41598-020-60172-6.
- ↑ 9.0 9.1 Pavlovic, Radmila; Panseri, Sara; Giupponi, Luca; Leoni, Valeria; Citti, Cinzia; Cattaneo, Chiara; Cavaletto, Maria; Giorgi, Annamaria (15 October 2019). "Phytochemical and Ecological Analysis of Two Varieties of Hemp (Cannabis sativa L.) Grown in a Mountain Environment of Italian Alps". Frontiers in Plant Science 10: 1265. doi:10.3389/fpls.2019.01265. ISSN 1664-462X. PMC PMC6822994. PMID 31708938. https://www.frontiersin.org/article/10.3389/fpls.2019.01265/full.
- ↑ Mastellone, Giulia; Marengo, Arianna; Sgorbini, Barbara; Scaglia, Federica; Capetti, Francesca; Gai, Francesco; Peiretti, Pier Giorgio; Rubiolo, Patrizia et al. (3 February 2022). "Characterization and Biological Activity of Fiber-Type Cannabis sativa L. Aerial Parts at Different Growth Stages" (in en). Plants 11 (3): 419. doi:10.3390/plants11030419. ISSN 2223-7747. PMC PMC8838183. PMID 35161400. https://www.mdpi.com/2223-7747/11/3/419.
- ↑ 11.0 11.1 11.2 Citti, Cinzia; Braghiroli, Daniela; Vandelli, Maria Angela; Cannazza, Giuseppe (1 January 2018). "Pharmaceutical and biomedical analysis of cannabinoids: A critical review" (in en). Journal of Pharmaceutical and Biomedical Analysis 147: 565–579. doi:10.1016/j.jpba.2017.06.003. https://linkinghub.elsevier.com/retrieve/pii/S0731708517311895.
- ↑ (in en) Recommended Methods for the Identification and Analysis of Cannabis and Cannabis Products. United Nations Office on Drugs and Crime. 6 March 2013. doi:10.18356/1e8e4f16-en. ISBN 978-92-1-055589-0. https://www.un-ilibrary.org/content/books/9789210555890.
- ↑ Lachenmeier, Dirk W.; Kroener, Lars; Musshoff, Frank; Madea, Burkhard (1 January 2004). "Determination of cannabinoids in hemp food products by use of headspace solid-phase microextraction and gas chromatography?mass spectrometry". Analytical and Bioanalytical Chemistry 378 (1): 183–189. doi:10.1007/s00216-003-2268-4. ISSN 1618-2642. http://link.springer.com/10.1007/s00216-003-2268-4.
- ↑ 14.0 14.1 14.2 14.3 Ilias, Yara; Rudaz, Serge; Mathieu, Patrick; Christen, Philippe; Veuthey, Jean-Luc (1 November 2005). "Extraction and analysis of differentCannabis samples by headspace solid-phase microextraction combined with gas chromatography-mass spectrometry" (in en). Journal of Separation Science 28 (17): 2293–2300. doi:10.1002/jssc.200500130. ISSN 1615-9306. https://onlinelibrary.wiley.com/doi/10.1002/jssc.200500130.
- ↑ 15.0 15.1 Czégény, Zsuzsanna; Nagy, Gréta; Babinszki, Bence; Bajtel, Ákos; Sebestyén, Zoltán; Kiss, Tivadar; Csupor-Löffler, Boglárka; Tóth, Barbara et al. (1 December 2021). "CBD, a precursor of THC in e-cigarettes" (in en). Scientific Reports 11 (1): 8951. doi:10.1038/s41598-021-88389-z. ISSN 2045-2322. PMC PMC8076212. PMID 33903673. http://www.nature.com/articles/s41598-021-88389-z.
- ↑ Psillakis, Elefteria (1 September 2017). "Vacuum-assisted headspace solid-phase microextraction: A tutorial review" (in en). Analytica Chimica Acta 986: 12–24. doi:10.1016/j.aca.2017.06.033. https://linkinghub.elsevier.com/retrieve/pii/S0003267017307390.
- ↑ Psillakis, Elefteria (1 September 2020). "The effect of vacuum: an emerging experimental parameter to consider during headspace microextraction sampling" (in en). Analytical and Bioanalytical Chemistry 412 (24): 5989–5997. doi:10.1007/s00216-020-02738-x. ISSN 1618-2642. https://link.springer.com/10.1007/s00216-020-02738-x.
- ↑ Psillakis, Elefteria; Mousouraki, Antonia; Yiantzi, Evangelia; Kalogerakis, Nicolas (1 June 2012). "Effect of Henry's law constant and operating parameters on vacuum-assisted headspace solid phase microextraction" (in en). Journal of Chromatography A 1244: 55–60. doi:10.1016/j.chroma.2012.05.006. https://linkinghub.elsevier.com/retrieve/pii/S002196731200708X.
- ↑ Psillakis, Elefteria; Yiantzi, Evangelia; Sanchez-Prado, Lucia; Kalogerakis, Nicolas (1 September 2012). "Vacuum-assisted headspace solid phase microextraction: Improved extraction of semivolatiles by non-equilibrium headspace sampling under reduced pressure conditions" (in en). Analytica Chimica Acta 742: 30–36. doi:10.1016/j.aca.2012.01.019. https://linkinghub.elsevier.com/retrieve/pii/S0003267012001195.
- ↑ 20.0 20.1 Capetti, Francesca; Rubiolo, Patrizia; Bicchi, Carlo; Marengo, Arianna; Sgorbini, Barbara; Cagliero, Cecilia (1 May 2020). "Exploiting the versatility of vacuum‐assisted headspace solid‐phase microextraction in combination with the selectivity of ionic liquid‐based GC stationary phases to discriminate Boswellia spp. resins through their volatile and semivolatile fractions" (in en). Journal of Separation Science 43 (9-10): 1879–1889. doi:10.1002/jssc.202000084. ISSN 1615-9306. https://onlinelibrary.wiley.com/doi/10.1002/jssc.202000084.
- ↑ Pollo, Breno J.; Romero-Orejón, Katherine L.; Marsaioli, Anita J.; Rosa, Paulo T.V.; Augusto, Fabio (1 February 2022). "Vacuum-assisted headspace solid-phase microextraction and gas chromatography coupled to mass spectrometry applied to source rock analysis" (in en). Advances in Sample Preparation 1: 100001. doi:10.1016/j.sampre.2021.100001. https://linkinghub.elsevier.com/retrieve/pii/S2772582021000012.
- ↑ Kolb, Bruno; Ettre, Leslie S. (2006). "Chapter 3: The technique of head-space-gas chromatography". Static headspace-gas chromatography: theory and practice (2nd ed ed.). Hoboken, N.J: Wiley. pp. 45–116. ISBN 978-0-471-74944-8.
- ↑ 23.0 23.1 "GC Calculators and Method Translation Software". Agilent Technologies, Inc.. https://www.agilent.com/en/support/gas-chromatography/gccalculators. Retrieved 29 January 2022.
- ↑ 24.0 24.1 24.2 Linstrom, Peter (1997), "NIST Chemistry WebBook, NIST Standard Reference Database 69", NIST Chemistry WebBook (National Institute of Standards and Technology), doi:10.18434/t4d303, https://webbook.nist.gov/chemistry/
- ↑ Bicchi, Carlo; Blumberg, Leonid; Cagliero, Cecilia; Cordero, Chiara; Rubiolo, Patrizia; Liberto, Erica (1 February 2010). "Development of fast enantioselective gas-chromatographic analysis using gas-chromatographic method-translation software in routine essential oil analysis (lavender essential oil)" (in en). Journal of Chromatography A 1217 (9): 1530–1536. doi:10.1016/j.chroma.2010.01.003. https://linkinghub.elsevier.com/retrieve/pii/S0021967310000142.
- ↑ Mazzucotelli, Maria; Minteguiaga, Manuel A.; Sgorbini, Barbara; Sidisky, Len; Marengo, Arianna; Rubiolo, Patrizia; Bicchi, Carlo; Cagliero, Cecilia (1 January 2020). "Ionic liquids as water-compatible GC stationary phases for the analysis of fragrances and essential oils: Quantitative GC–MS analysis of officially-regulated allergens in perfumes" (in en). Journal of Chromatography A 1610: 460567. doi:10.1016/j.chroma.2019.460567. https://linkinghub.elsevier.com/retrieve/pii/S0021967319309616.
- ↑ "EPI Suite - Estimation Program Interface". U.S. Environmental Protection Agency. 18 January 2022. https://www.epa.gov/tsca-screening-tools/epi-suitetm-estimation-program-interface. Retrieved 02 February 2022.
- ↑ 28.0 28.1 Lord, Heather; Pawliszyn, Janusz (1 July 2000). "Evolution of solid-phase microextraction technology" (in en). Journal of Chromatography A 885 (1-2): 153–193. doi:10.1016/S0021-9673(00)00535-5. https://linkinghub.elsevier.com/retrieve/pii/S0021967300005355.
- ↑ Risticevic, Sanja; Lord, Heather; Górecki, Tadeusz; Arthur, Catherine L; Pawliszyn, Janusz (1 January 2010). "Protocol for solid-phase microextraction method development" (in en). Nature Protocols 5 (1): 122–139. doi:10.1038/nprot.2009.179. ISSN 1754-2189. http://www.nature.com/articles/nprot.2009.179.
- ↑ Yiantzi, Evangelia; Kalogerakis, Nicolas; Psillakis, Elefteria (1 August 2015). "Vacuum-assisted headspace solid phase microextraction of polycyclic aromatic hydrocarbons in solid samples" (in en). Analytica Chimica Acta 890: 108–116. doi:10.1016/j.aca.2015.05.047. https://linkinghub.elsevier.com/retrieve/pii/S0003267015007989.
- ↑ Trujillo-Rodríguez, María J.; Pino, Verónica; Psillakis, Elefteria; Anderson, Jared L.; Ayala, Juan H.; Yiantzi, Evangelia; Afonso, Ana M. (1 April 2017). "Vacuum-assisted headspace-solid phase microextraction for determining volatile free fatty acids and phenols. Investigations on the effect of pressure on competitive adsorption phenomena in a multicomponent system" (in en). Analytica Chimica Acta 962: 41–51. doi:10.1016/j.aca.2017.01.056. https://linkinghub.elsevier.com/retrieve/pii/S0003267017301472.
Notes
This presentation is faithful to the original, with only a few minor changes to presentation. Some grammar and punctuation was cleaned up to improve readability. In some cases important information was missing from the references, and that information was added. Nothing else was changed in accordance with the NoDerivatives portion of the license.
- Pages containing cite templates with deprecated parameters
- LIMSwiki journal articles (added in 2022)
- LIMSwiki journal articles (all)
- LIMSwiki journal articles on cannabis cannabinoids
- LIMSwiki journal articles on cannabis terpenes
- LIMSwiki journal articles on cannabis testing
- LIMSwiki journal articles (with rendered math)