Difference between revisions of "Journal:A survival guide for the rapid transition to a fully digital workflow: The Caltagirone example"
Shawndouglas (talk | contribs) (Saving and adding more.) |
Shawndouglas (talk | contribs) (Saving and adding more.) |
||
| Line 174: | Line 174: | ||
|- | |- | ||
|} | |} | ||
Two monitors with different roles have been connected to each computer, allowing the simultaneous evaluation of the case page in the LIS and the respective WSI from different displays. As per manufacturer instructions, the employed LIS required a minimum 17-inch monitor to run, and the department introduced devices with a range of 17 to 27 inches. On the other hand, the monitors dedicated to the visualization of the WSI were 24 to 27 inches in size (Table 3). | |||
{| class="wikitable" border="1" cellpadding="5" cellspacing="0" width="60%" | |||
|- | |||
| colspan="5" style="background-color:white; padding-left:10px; padding-right:10px;" |'''Table 3.''' Specifications of monitors used for WSI visualization | |||
|- | |||
! style="background-color:#dddddd; padding-left:10px; padding-right:10px;" |Manufacturer | |||
! style="background-color:#dddddd; padding-left:10px; padding-right:10px;" |Size | |||
! style="background-color:#dddddd; padding-left:10px; padding-right:10px;" |Resolution | |||
! style="background-color:#dddddd; padding-left:10px; padding-right:10px;" |Type | |||
! style="background-color:#dddddd; padding-left:10px; padding-right:10px;" |Refresh rate (Hz) | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Hannstar | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |23.6 inch | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |1920 × 1080 pixels | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |LCD | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |60 | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Philips | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |27 inch | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |1920 × 1080 pixels | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |LED | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |75 | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Fujitsu | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |27 inch | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |2560 × 1440 pixels | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |LCD | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |60 | |||
|- | |||
|} | |||
WSIs were directly accessed from the AP-LIS. Specifically, a virtual slide tray was created and incorporated within the AP-LIS, as described previously. [6] Accessioning of cases and real-time tracking of digital slides occurred directly from the AP-LIS. The creation of a single slide tray within the AP-LIS Pathox, displaying the macroimage (thumbnail) of several slides simultaneously, allowed the incorporation of WSI acquired from the Pannoramic 250 Flash III scanner, with the possibility to connect different scanners from different vendors (by using the image management system of the scanner as a simple [[middleware]]) without disrupting the end-user workflow. All images were saved on network-attached storage (96 TB Qnap NAS TVS-EC1280U-SAS-RP) using the dedicated 100 Mbps network connection. All the scanned slides were stored in the server as a digital database of WSIs, allowing a possible retrospective consultation directly from the AP-LIS. The eventual re-scan of a slide resulted in the overwriting of the previously scanned one, so that pathologists always had access to the most recent images. Validation of the WSIs for their use for primary histological diagnosis was made according to College of American Pathologists (CAP) guidelines. [17] | |||
==Results== | |||
In 2019, just before the advent of COVID-19, the Caltagirone pathology department had a yearly workload of 8,182 histological cases with a total of 42,245 corresponding slides. The entire activity of the laboratory was modified, starting from the limitations and issues related to the previous analog and non-tracked workflow, following different steps (checkpoints), as reported below. This allowed a complete transition towards a digital pathology approach, leading to the digital primary sign-out of all the cases through WSIs. Before the implementation of the digital workflow, no standard procedures and checkpoints were present along the different steps of sample processing. Addressing these deficiencies was mandatory for the full digital transition, in order to have a more efficient fully tracked and paperless workflow. Here we report the introduced checkpoints at every step of the specimen handling that should be followed to obtain a fully integrated system (Table 4). | |||
{| class="wikitable" border="1" cellpadding="5" cellspacing="0" width="60%" | |||
|- | |||
| colspan="3" style="background-color:white; padding-left:10px; padding-right:10px;" |'''Table 4.''' Different steps of specimen processing as they are performed before and after the implementation of digital pathology in the laboratory. DP: digital pathology; LIS: laboratory information system; WSI: whole slide image. | |||
|- | |||
! style="background-color:#dddddd; padding-left:10px; padding-right:10px;" |Checkpoint | |||
! style="background-color:#dddddd; padding-left:10px; padding-right:10px;" |Before DP implementation | |||
! style="background-color:#dddddd; padding-left:10px; padding-right:10px;" |After DP implementation | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Accessioning checkpoint | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |• Paper request with handwritten patient and specimen data<br /> <br />• Manual check for correspondence between request paper and label on the specimen<br /> <br />• Manual insertion of the case in the AP-LIS or (worse) new internal “working paper” generated to accompany the specimen in the different subsequent phases of the process (lab sheet) | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |• Order entry system, patient-identifying barcode, case, and specimen container information imported from the integrated hospital LIS with digital request (no more transcription errors)<br /> <br />• Progressive number linked to the barcode generated and used for all sorts of assets generated for that case (tracking of the sample through its journey in the laboratory)<br /> <br />• The administrator will take a picture of the container and of the specimen and those photos will be attached to the case file (medico-legal registry)<br /> <br />• Documents attached to the specimen are scanned and attached to the case file (relevant information handy) | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Grossing checkpoint | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |• The grossing operator (e.g., pathologist) has the working paper (lab sheet) as the only reference to the case<br /> <br />• No pictures of the sample as it is when it arrives at the grossing room are taken<br /> <br />• Manual transcription of macroscopic description of the sample by the pathologist or the assistant technician (dictation/transcription errors)<br /> <br />• Cassettes are labeled manually by the pathologist/technician | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |• Automatic access to the case by scanning the identification barcode on the sample container<br /> <br />• Photographic documentation of different grossing steps (specimen in the container, during grossing and within the cassettes) guarantees the preservation of the case features and identification<br /> <br />• Direct dictation of the macroscopic description of the sample converted to text through voice recognition functions of the LIS<br /> <br />• Cassettes are printed with the identification code of the sample to be tracked in further workstations | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Sectioning checkpoint | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |• The number transcribed by the grossing operator on the block is copied on the slide, becoming a possible source of errors<br /> <br />• The needed stain for each case is reported on the working paper (lab sheet) or indicated by the color of the cassette<br /> <br />• The generated slides are manually transcribed by the sectioning technician, and no barcode is printed on the slide<br /> <br />• After the sectioning phase, the block is archived and no pictures of the cut surface of the block are taken<br /> <br />• The sectioning phase lacks strict quality criteria; the presence of artifacts, folding, inappropriate coverslipping does not significantly impair the physical microscope visualization | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |• The code printed on the paraffin block may be scanned to open the case file through the integrated LIS, preventing transcription errors<br /> <br />• The technician can check how many and which kind of slides are needed for each block directly on the LIS<br /> <br />• For each paraffin block, one or more printed glass slides are generated through a dedicated printer, including the identification code<br /> <br />• After sectioning, each paraffin block may be photographed to assess whether all the material emerged on the glass slide/WSI<br /> <br />• Sectioning phase should follow high operative standards, reducing the risk of artifacts that can impair the scanning phase | |||
|- | |||
|} | |||
Revision as of 18:05, 1 February 2022
| Full article title | A survival guide for the rapid transition to a fully digital workflow: The Caltagirone example |
|---|---|
| Journal | Diagnostics |
| Author(s) | Fraggetta, Filippo; Caputo, Alessandro; Guglielmino, Rosa; Pellegrino, Maria G.; Runza, Giampaolo; L'Imperio, Vincenzo |
| Author affiliation(s) | Ospedale Gravina, University of Salerno, University of Milano-Bicocca |
| Primary contact | Email: filippofra at hotmail dot com |
| Editors | Crescenzi, Anna |
| Year published | 2021 |
| Volume and issue | 11(10) |
| Article # | 1916 |
| DOI | 10.3390/diagnostics11101916 |
| ISSN | 2075-4418 |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://www.mdpi.com/2075-4418/11/10/1916/htm |
| Download | https://www.mdpi.com/2075-4418/11/10/1916/pdf (PDF) |
|
|
This article should be considered a work in progress and incomplete. Consider this article incomplete until this notice is removed. |
Abstract
Digital pathology for the routine assessment of cases for primary diagnosis has been implemented by few laboratories worldwide. The Gravina Hospital in Caltagirone (Sicily, Italy), which collects cases from seven different hospitals distributed in the Catania area, converted its entire workflow to digital starting from 2019. Before the transition, the Caltagirone pathology laboratory was characterized by a non-tracked workflow, based on paper requests, and hand-written blocks and slides, as well as manual assembling and delivering of the cases and glass slides to the pathologists. Moreover, the arrangement of the spaces and offices in the department was illogical and under-productive for the linearity of the workflow. For these reasons, an adequate 2D barcode system for tracking purposes, the redistribution of laboratory spaces, and the implementation of whole-slide imaging (WSI) technology based on a laboratory information system (LIS)-centric approach were adopted as a needed prerequisite to switch to a digital workflow. The adoption of a dedicated connection for transfer of clinical and administrative data between different software and interfaces using an internationally recognized standard (Health Level 7) in the pathology department further facilitated the transition, helping in the integration of the LIS with WSI scanners. As per previous reports, the components and devices chosen for the pathologists’ workstations did not significantly impact on the WSI-based reporting phase in primary histological diagnosis. An analysis of all the steps of this transition has been made retrospectively to provide a useful “handy” guide to lead the digital transition of “analog,” non-tracked pathology laboratories following the experience of the Caltagirone pathology department. Following step-by-step instructions towards the implementation of a paperless routine with more standardized and safe processes, every "analog" pathology department has the real possibility of managing case priority and implementing artificial intelligence (AI) tools.
Keywords: digital pathology, WSI, LIS, 2D barcode, primary diagnosis
Introduction
A progressively increasing number of pathology departments are deploying, or planning to deploy, digital pathology systems for all or part of their diagnostic output. [1,2,3,4,5] Some of the present work's authors have already experienced the full transition to a digital workflow [6], eventually upgrading scanning procedures at the magnification of 40× and even integrating artificial intelligence (AI) tools for the assessment of specific specimens (e.g., prostate biopsies) in routine practice. [7] Moreover, the employment of a secure virtual private network (VPN) connection has allowed other pathologists to work off-site [8], significantly helping during the recent COVID-19 pandemic. [9]
However, despite this revolutionary transition, real world data suggest that a fully digital approach to the histological workflow has been implemented in only a minority of pathology laboratories, in Italy as well as worldwide. Several reasons have been advocated to explain what is holding us to the traditional “analog” workflow. [10] Although some major benefits of the digital approach (e.g., safety, quality, efficiency, easy and equal access to expert pathologists/second opinions) are widely recognized, some aspects of digital pathology may still cause reluctance within the pathology community, including the costs, the lack of validation data, and the possible threat represented by this kind of implementation for the pathologists. [11]
Moreover, the Food and Drug Administration (FDA) approved some but not all of the available scanning systems (Philips and Leica) for digital primary diagnosis. The current lack of approval for all the other devices (e.g., 3DHistech, Hamamatsu, Ventana, etc.) is further slowing down the transition, even in the United States where a widespread implementation of a fully digital workflow using whole-slide imaging (WSI) for primary diagnosis is still in progress.
All these components contribute to the generalized skepticism of the pathologists towards these innovative paradigms, at least partly explaining the slow implementation of digital pathology in routine. The adoption of the advocated workflow is further complicated by the substantial lack of an adequate tracking system based on linear or 2D barcodes in the majority of the laboratories, which could represent an obstacle to benefiting from all the advantages of the digital transition. [11]
Based on the previously reported Catania experience at the Cannizzaro Hospital [6], this paper shows the step-by-step process followed by the pathology department of the Gravina Hospital in Caltagirone (Sicily, Italy) to switch from a non-tracked system to a fully digital workflow in a few months, embracing all the benefits of digital pathology.
This may exemplify a simple and efficient transition from glass slides to WSI, thanks to the logical implementations made in the pathology laboratory of Caltagirone, in which the introduction of slide scanners represents only the last intuitive step of a complete digital workflow. Our experience is reported to the benefit of the numerous laboratories planning or working to implement digital pathology.
Materials and methods
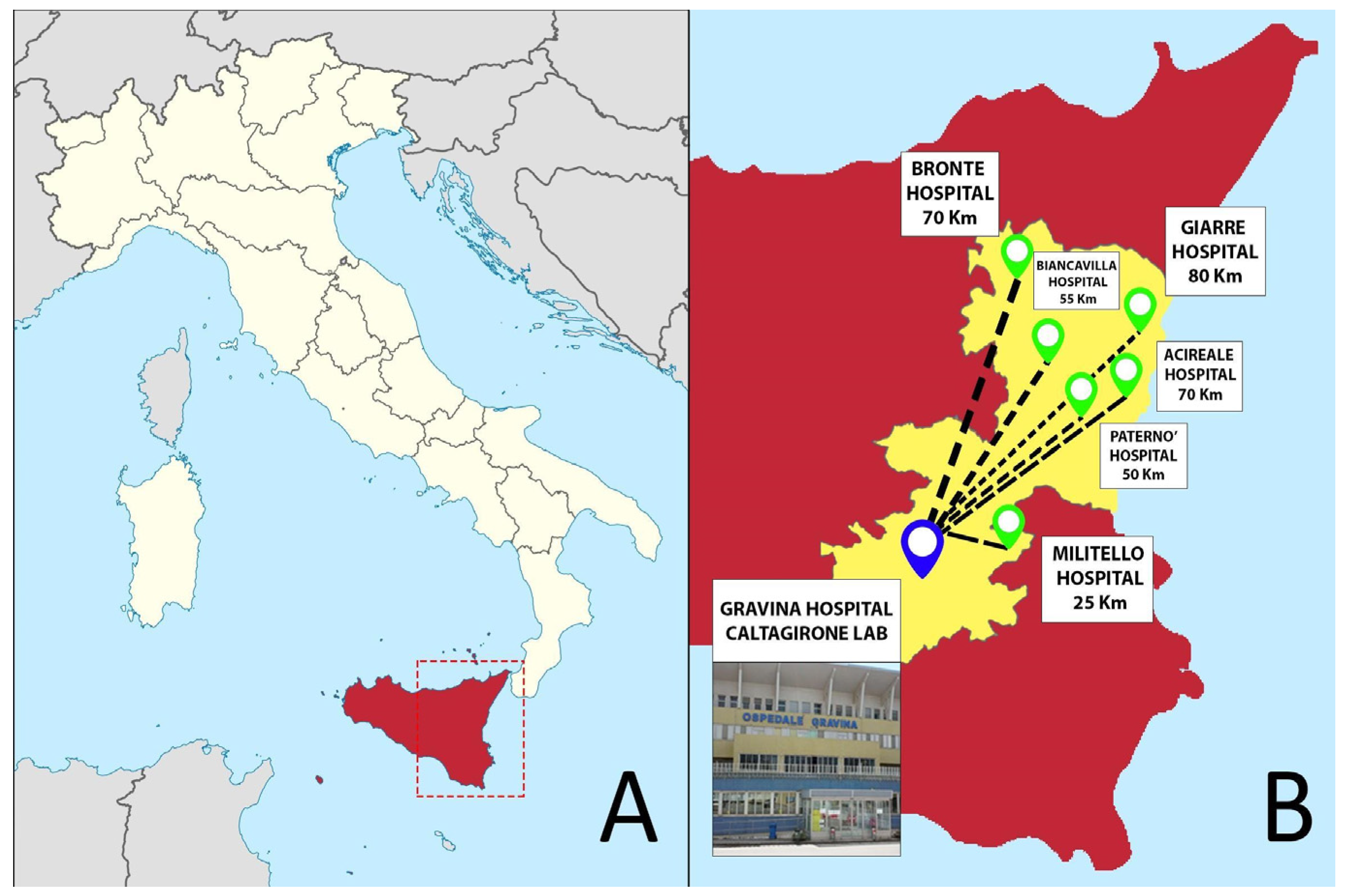
Gravina Hospital represents the pathology laboratory hub of the Azienda Sanitaria Provinciale (ASP) of Catania in Sicily (south of Italy), collecting specimens—mainly surgical and bioptic—from seven different hospitals distributed in the Catania area (Figure 1). Starting from 2019, the pathology department of Caltagirone experienced a profound transformation that required about four months to switch from a non-tracked, conventional pathology workflow to a fully digital approach. Similar to the previous Catania experience [6], the entire workflow was converted into a digital one, while also introducing some additional digital checkpoints through the different steps of the process.
|
To allow and facilitate this transition, the following implementations were needed:
- lean workflow and rearrangement of spaces and offices;
- implementation of the information technology infrastructure;
- implementation of the tracking system and checkpoint procedures;
- implementation of the automation components; and
- implementation of the scanning components.
Lean workflow and rearrangement of spaces and offices
In order to achieve the best result of digitization, we first solved some logistic problems in the lab. Following the lean laboratory philosophy [12,13], laboratory spaces were rearranged. This started from a redistribution of the rooms in a linear manner based on the natural sequence of the processing steps. This significantly reduced the personnel and specimen transfers and optimized the working time through the arrangement of similar tasks (e.g., staining and scanning) in the same room and through the creation of inter-room communications. Thanks to a better distribution of the spaces, these modifications freed two rooms that were used to create the molecular diagnostics section, previously absent in the lab partly due to the inefficient disposition of the spaces.
Implementation of the information technology infrastructure
Before implementation of the digital workflow, a dedicated network and servers to store the images and the linked metadata were lacking in the lab. As a consequence, the spaces and offices were not equipped with the necessary access points for the network, and the different instruments used for the analog workflow were not interconnected through the laboratory information system (LIS). Thus, along with the adoption of a lean approach to the workflow, we implemented the information technology infrastructure, which consisted of the creation of internet access points (network access) based on the position and type of instruments to be connected and a dedicated bandwidth of 100 Mbps.
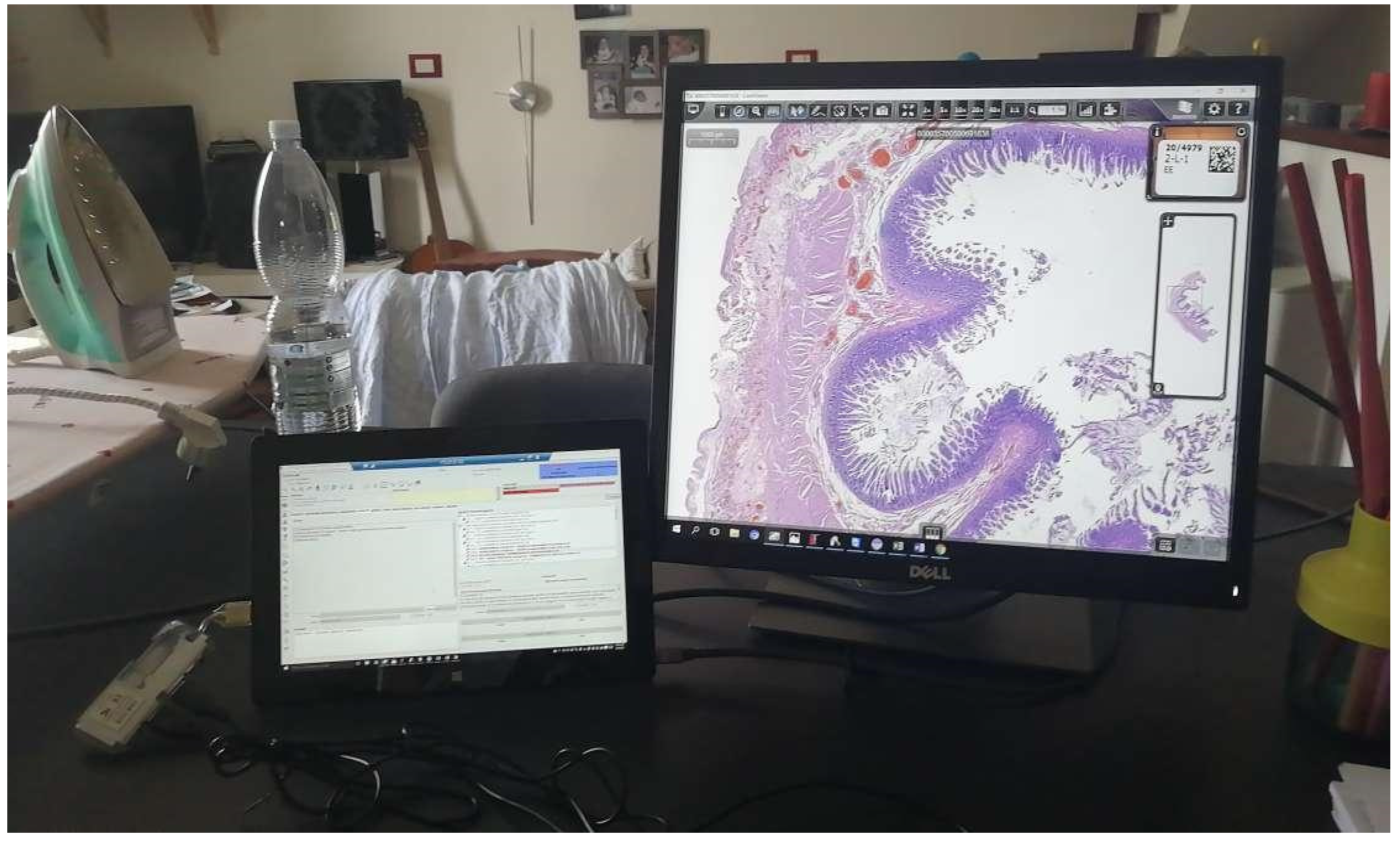
The entire digital workflow switch has been centered on the implementation of an anatomic pathology LIS (AP-LIS), Pathox (version 13.22.0, Tesi Elettronica e Sistemi Informativi SpA, Milan, Italy), allowing the integration of the case/specimen information from the accessioning to the reporting phases. The majority of the instruments present in the lab were integrated with the LIS using the 2D barcode system with interface exchanges handled through Health Level 7 (HL7) version 2.5 messages. Based on the previous experience [6], the integration took only a few days of work (including the implementation of the scanner, which took two days). This is in contrast to other reported similar implementations that required more time to deploy. [14,15] Furthermore, the implementation of a secure VPN connection allowed the pathologists to access and report cases from home (Figure 2).
|
Implementation of the tracking system and checkpoint procedures
The lab lacked a proper tracking system, and information on the tissue blocks and glass slides was handwritten. Not all the steps of the workflow were appropriately tracked (i.e., gross examination, tissue processing, and paraffin-embedding), and different/redundant paper sheets accompanied the workflow from the accessioning to the assembling and delivering of the glass slides for each phase. This analog workflow was abandoned in favor of a new paperless 2D-barcode tracking system, fully integrated with the LIS.
This new system was then implemented through the entire workflow, from accessioning to diagnosis. Two-dimensional barcodes were preferred to one-dimensional ones because they are less space-demanding (fitting well on the tiny surface of both tissue blocks and glass slides), more easily applicable to the convex surfaces of tissue containers, and generally less prone to scanning issues. Moreover, we introduced laser printers for blocks in order to obtain a permanent mark of the barcode on the surfaces (see the Grossing subsection of the Results section). The implementation of the tracking system within the LIS gave us the possibility to safely and efficiently monitor every step of the workflow through the use of dashboards.
Implementation of the automation components
To promote automation of processes following the lean philosophy, some instrumental implementations were introduced in the laboratory, simplifying many laboratory procedures that were previously performed manually and in a repetitive manner (Table 1). All of these achievements were made possible mainly thanks to the HL7 connection and the widespread use of 2D barcodes. However, the prototype of automation was represented by the automatic assembling and delivering of the slides through the use of scanning systems together with the 2D barcode–based archiving of blocks and slides. Finally, the immunohistochemistry instrument (Autostainer Link 48, Agilent, Santa Clara, CA, USA) was completely interconnected with the LIS (Pathox) through the HL7 connection.
| Table 1. Automation introduced at every step of the workflow | |
| Phase | Automation introduced |
|---|---|
| Accessioning | Adoption of order entry and A4 flat scanner to digitize all the paper documents associated with the cases (i.e., endoscopic exams, clinical annotations, etc.) |
| Grossing | Introduction of a laser block printer and camera at the grossing bench, enabling the possibility to capture the material in the block |
| Processing/embedding | Introduction of a real-time multi-barcode scanner, enabling the possibility of matching blocks produced at grossing with those sent to processing |
| Sectioning | Allow for the possibility to capture the cut surface of the block for review purposes, as well as the automated printing of barcodes directly on glass slides rather than on labels |
| Staining | Allow for the automation of requests of histochemical and immunohistochemical stains, which are delivered directly to the stainer |
| Archiving | Improvement of the archiving of slides and blocks, whose position in the storage trays is random and tracked automatically by barcode scanning |
Implementation of the scanning components
Since the main paradigm chosen for the digital workflow switch was based on the LIS-centric philosophy, this allowed a perfect integration of different scanning platforms independently from the vendor, the WSI formats (e.g., .tiff, .svs, .vms, .ndpi) and the provided platform for slides visualization. This change in the paradigm did not force the department to employ a specific scanner device, leading to choose a fast (35 seconds per slide) and high throughput (60 slides per hour) scanner (Pannoramic 250 flash III, 3DHistech, Budapest, Hungary), with a load capacity of 300 slides and good performances with brightfield and darkfield applications. The digitization involved standard hematoxylin and eosin (H&E) slides, as well as special histochemical, immunohistochemical, and immunofluorescence slides (for both conventional immunofluorescence and fluorescence in situ hybridization [FISH]). For the frozen sections and intraoperative procedures, the Aperio LV1 IVD system (Leica Biosystem, Nussloch, Germany) was employed due to its ability to obtain live images from up to four slides with magnification up to 63×. Digitizing cytology slides was not undertaken due to the need for Z-stack image acquisition, which increases scan time and file size. [16] The scanning system was operated by technicians who were trained to use these devices to support routine daily work. To further optimize the workflow, the scanning station was located in the same room where slides were stained, coverslipped, and prepared for archiving (Stainer AUS 240, Bio-optica, Milan, Italy; Leica Coverslipper CV5030, Leica Biosystems, Nussloch, Germany). Regular maintenance was performed every month taking into account white/color balance and adjustment of the scanner focus.
After the scanning process, the slides were automatically assigned to the proper cases and virtually delivered to the pathologists. [6] The slides appeared in the virtual tray within the LIS, and cases with scanning completed for all the slides belonging to them were considered ready to be reported.
Pathologists’ workstations were composed of one computer with two monitors. Different computer devices have been implemented for the pathologists’ workstations (Table 2), with central processor units (CPU) of different generations, different clock speed and vendors (Intel and AMD), and random access memory (RAM) with different size (4 and 8 GB), as well as various video cards, mostly integrated.
| Table 2. The different computer devices employed in the Caltagirone digital pathology lab for the pathologists’ workstations. CPU: central processing unit; RAM: random-access memory; OS: operating system; W10: Windows 10 (Microsoft, Redmond, WA, USA). | ||||
| CPU | Clock speed | RAM | OS | Dedicated video |
|---|---|---|---|---|
| AMD Ryzen 5Pro 2400 G | 3.60 GHz | 8 GB | W10 64 bit | none (CPU-integrated) |
| Intel Core i3-9100 | 3.60 GHz | 8 GB | W10 64 bit | none (CPU-integrated) |
| Intel Core i7-8700 | 3.20 GHz | 8 GB | W10 64 bit | none (CPU-integrated) |
| Intel Core i5-4590 | 3.30 GHz | 8 GB | W10 64 bit | none (CPU-integrated) |
| Intel Core i3-2120 | 3.30 GHz | 4 GB | W10 64 bit | none (CPU-integrated) |
| Intel Celeron 3865U | 1.80 GHz | 8 GB | W10 64 bit | none (CPU-integrated) |
Two monitors with different roles have been connected to each computer, allowing the simultaneous evaluation of the case page in the LIS and the respective WSI from different displays. As per manufacturer instructions, the employed LIS required a minimum 17-inch monitor to run, and the department introduced devices with a range of 17 to 27 inches. On the other hand, the monitors dedicated to the visualization of the WSI were 24 to 27 inches in size (Table 3).
| Table 3. Specifications of monitors used for WSI visualization | ||||
| Manufacturer | Size | Resolution | Type | Refresh rate (Hz) |
|---|---|---|---|---|
| Hannstar | 23.6 inch | 1920 × 1080 pixels | LCD | 60 |
| Philips | 27 inch | 1920 × 1080 pixels | LED | 75 |
| Fujitsu | 27 inch | 2560 × 1440 pixels | LCD | 60 |
WSIs were directly accessed from the AP-LIS. Specifically, a virtual slide tray was created and incorporated within the AP-LIS, as described previously. [6] Accessioning of cases and real-time tracking of digital slides occurred directly from the AP-LIS. The creation of a single slide tray within the AP-LIS Pathox, displaying the macroimage (thumbnail) of several slides simultaneously, allowed the incorporation of WSI acquired from the Pannoramic 250 Flash III scanner, with the possibility to connect different scanners from different vendors (by using the image management system of the scanner as a simple middleware) without disrupting the end-user workflow. All images were saved on network-attached storage (96 TB Qnap NAS TVS-EC1280U-SAS-RP) using the dedicated 100 Mbps network connection. All the scanned slides were stored in the server as a digital database of WSIs, allowing a possible retrospective consultation directly from the AP-LIS. The eventual re-scan of a slide resulted in the overwriting of the previously scanned one, so that pathologists always had access to the most recent images. Validation of the WSIs for their use for primary histological diagnosis was made according to College of American Pathologists (CAP) guidelines. [17]
Results
In 2019, just before the advent of COVID-19, the Caltagirone pathology department had a yearly workload of 8,182 histological cases with a total of 42,245 corresponding slides. The entire activity of the laboratory was modified, starting from the limitations and issues related to the previous analog and non-tracked workflow, following different steps (checkpoints), as reported below. This allowed a complete transition towards a digital pathology approach, leading to the digital primary sign-out of all the cases through WSIs. Before the implementation of the digital workflow, no standard procedures and checkpoints were present along the different steps of sample processing. Addressing these deficiencies was mandatory for the full digital transition, in order to have a more efficient fully tracked and paperless workflow. Here we report the introduced checkpoints at every step of the specimen handling that should be followed to obtain a fully integrated system (Table 4).
| Table 4. Different steps of specimen processing as they are performed before and after the implementation of digital pathology in the laboratory. DP: digital pathology; LIS: laboratory information system; WSI: whole slide image. | ||
| Checkpoint | Before DP implementation | After DP implementation |
|---|---|---|
| Accessioning checkpoint | • Paper request with handwritten patient and specimen data • Manual check for correspondence between request paper and label on the specimen • Manual insertion of the case in the AP-LIS or (worse) new internal “working paper” generated to accompany the specimen in the different subsequent phases of the process (lab sheet) |
• Order entry system, patient-identifying barcode, case, and specimen container information imported from the integrated hospital LIS with digital request (no more transcription errors) • Progressive number linked to the barcode generated and used for all sorts of assets generated for that case (tracking of the sample through its journey in the laboratory) • The administrator will take a picture of the container and of the specimen and those photos will be attached to the case file (medico-legal registry) • Documents attached to the specimen are scanned and attached to the case file (relevant information handy) |
| Grossing checkpoint | • The grossing operator (e.g., pathologist) has the working paper (lab sheet) as the only reference to the case • No pictures of the sample as it is when it arrives at the grossing room are taken • Manual transcription of macroscopic description of the sample by the pathologist or the assistant technician (dictation/transcription errors) • Cassettes are labeled manually by the pathologist/technician |
• Automatic access to the case by scanning the identification barcode on the sample container • Photographic documentation of different grossing steps (specimen in the container, during grossing and within the cassettes) guarantees the preservation of the case features and identification • Direct dictation of the macroscopic description of the sample converted to text through voice recognition functions of the LIS • Cassettes are printed with the identification code of the sample to be tracked in further workstations |
| Sectioning checkpoint | • The number transcribed by the grossing operator on the block is copied on the slide, becoming a possible source of errors • The needed stain for each case is reported on the working paper (lab sheet) or indicated by the color of the cassette • The generated slides are manually transcribed by the sectioning technician, and no barcode is printed on the slide • After the sectioning phase, the block is archived and no pictures of the cut surface of the block are taken • The sectioning phase lacks strict quality criteria; the presence of artifacts, folding, inappropriate coverslipping does not significantly impair the physical microscope visualization |
• The code printed on the paraffin block may be scanned to open the case file through the integrated LIS, preventing transcription errors • The technician can check how many and which kind of slides are needed for each block directly on the LIS • For each paraffin block, one or more printed glass slides are generated through a dedicated printer, including the identification code • After sectioning, each paraffin block may be photographed to assess whether all the material emerged on the glass slide/WSI • Sectioning phase should follow high operative standards, reducing the risk of artifacts that can impair the scanning phase |
References
Notes
This presentation is faithful to the original, with only a few minor changes to presentation, spelling, and grammar. In some cases important information was missing from the references, and that information was added.