Journal:Conversion of a classical microbiology laboratory to a total automation laboratory enhanced by the application of lean principles
| Full article title | Conversion of a classical microbiology laboratory to a total automation laboratory enhanced by the application of lean principles |
|---|---|
| Journal | Microbiology Spectrum |
| Author(s) | Trigueiro, Graça; Oliveira, Carlos; Rodrigues, Alexandra; Seabra, Sofia; Pinto, Rui; Bala, Yohann; Granado, Monica G.; Vallejo, Sandra; Gonzalez, Victoria; Cardoso, Carlos |
| Author affiliation(s) | Dr. Joaquim Chaves Clinical Analysis Laboratory, bioMérieux |
| Primary contact | Email: alexandra dot cristina at jcs dot pt |
| Editors | Doucet-Populaire, Florence C. |
| Year published | 2024 |
| Volume and issue | 12(2) |
| Article # | e02153-23 |
| DOI | 10.1128/spectrum.02153-23 |
| ISSN | 2165-0497 |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://journals.asm.org/doi/10.1128/spectrum.02153-23 |
| Download | https://journals.asm.org/doi/pdf/10.1128/spectrum.02153-23?download=true (PDF) |
|
|
This article should be considered a work in progress and incomplete. Consider this article incomplete until this notice is removed. |
Abstract
Laboratory automation in microbiology improves productivity and reduces sample turnaround times (TATs). However, its full potential can be unlocked through the optimization of workflows by adopting lean principles. This study aimed to explore the relative impact of laboratory automation and continuous improvement events (CIEs) on productivity and TATs. Laboratory automation took place in November 2020 and consisted of the introduction of WASPLab and VITEK MS systems. CIEs were run in May and September 2021. Before the conversion, the laboratory processed approximately 492 samples on weekdays and had 10 full-time equivalent (FTE) staff for a productivity of 49 samples/FTE/day. In March 2021, after laboratory automation, the caseload went up to approximately 621 while the FTEs decreased to 8.5, accounting for a productivity improvement to 73 samples/FTE/day. The hypothetical productivity went up to 110 samples/FTE/day following CIEs, meaning that the laboratory could at that point deal with a caseload increase to approximately 935 with unchanged FTEs. Laboratory conversion also led to an improvement in TATs for all sample types. For vaginal swabs and urine samples, median TATs decreased from 70.3 hours (interquartile range [IQR]: 63.5–93.1) and 73.7 hours (IQR: 35.6–50.7) to 48.2 hours (IQR: 44.8–67.7) and 40.0 hours (IQR: 35.6–50.7), respectively. Automation alone was responsible for 37.2% and 75.8% of TAT reduction, respectively, while the remaining reduction of 62.8% and 24.2%, respectively, was achieved due to CIEs. The laboratory reached productivity and TAT goals predefined by the management after CIEs. In conclusion, automation substantially improved productivity and TATs, while the subsequent implementation of lean management further unlocked the potential of laboratory automation.
Importance
In this study, we combined total laboratory automation with lean management to show that appropriate laboratory work organization enhanced the benefit of the automation and substantially contributed to productivity improvements. Globally, the rapid availability of accurate results in the setting of a clinical microbiology laboratory is part of patient-centered approaches to treat infections and helps the implementation of antibiotic stewardship programs backed by the World Health Organization (WHO). Locally, from the point of view of laboratory management, it is important to find ways of maximizing the benefits of the use of technology, as total laboratory automation is an expensive investment.
Keywords: turnaround time, productivity, lean principles, laboratory automation, change management
Introduction
Compared to general hematology and biochemistry laboratories, automation was late to arrive in microbiology laboratories because of the complexity of the processes involved. [1–3] The most important driver of automation adoption in microbiology laboratories in recent times is represented by a shift of paradigm in clinical microbiology that increased the demand for rapid and accurate results. [1, 2, 4] The availability of such results is important for the success of patient-centered approaches to treat infections and for antibiotic stewardship programs. [5] Over the past 20 years, and especially since the onset of the coronavirus disease 2019 (COVID-19) pandemic, microbiology laboratories have been increasingly adopting technologies and processes that improve quality and efficiency, alongside a reduction in sample turnaround times (TATs). [1]
In total laboratory automation systems, all main system components (i.e., plating/streaking unit, incubation unit, high-resolution imaging system, colony picking for identification and antimicrobial susceptibility testing, and post-imaging analysis workstation) are robot driven. Artificial intelligence (AI) has recently been incorporated into imaging analysis to automatically interpret plates. Automating analyses has been the key to improving productivity. [6–8] System components may be connected via a conveyor belt system or placed in convenient locations in laboratories. [9] In the WASPLab system, plates are sorted out to stackers and transferred to workstations by technologists. The systems must be flexible enough to accommodate several variables, such as specimen types and non-standardized containers. Moreover, some samples are sent to be screened for a specific pathogen (e.g., throat swab samples to be screened for Streptococcus pyogenes), while others may require a variety of microbial culture and identification methods for a full diagnostic work-up. [1]
There are numerous advantages to total laboratory automation. Although a high investment is needed for the conversion from a manual to an automated lab, in the long run, automated laboratories are cheaper to run. [4] The implementation of total laboratory automation increases efficiency, measured as turnaround time and total productivity, and improves the quality of testing, thanks to the standardization and optimization of the procedures. Automation offers a possibility of better sample management and traceability, often requires lower volumes of samples, renders laboratory accreditation easier to obtain, and lowers the biological risk to the operators. [2, 10] In the context of a microbiology laboratory, a significantly better recovery of pathogens—defined as a greater number of unique colony morphologies, a higher proportion of discrete colonies, and the identification of more species/plates—can be achieved, as described by Bailey et al. [1]
However, there are two principal elements of laboratory automation: hardware and workflow. [1] Total laboratory automation improves efficiency through the reduction of repetitive tasks with moderate added value, i.e., through the modification of the workflow. Automated incubators with digital imaging drastically reduce the number of manipulations of the culture media plates. About 97% of samples are suitable for automatic processing. [2] Employees often resist change. Fear of the unknown, lack of trust toward leaders, comfort derived from a familiar routine, lack of perception that a change is needed, perceived lack of knowledge or competence and the necessity to retrain, poor communication of what will happen from the management, and exhaustion or saturation when changes are too frequent are all factors at the basis of resistance to change. [12] Therefore, to be successful, the introduction of a change must be “managed” through a process known as change management. Change management occurs through steps (i.e., prepare, implement, monitor, sustain, and reevaluate). [12] In particular, a continuous improvement in streamlining workflow must be introduced. Change management is done with the use of lean methodology, an evidence-based approach to increase quality and efficiency that combines philosophy, processes, people, and structures. [13]
The study aimed to assess the relative impact of laboratory automation, change management, and continuous improvement events (CIEs) on key performance indicators (KPIs, i.e., full-time equivalent [FTE]/day and TAT) in a clinical microbiology laboratory concerning the processing of urine samples, vaginal swabs, and stool samples that comprise over 90% of the caseload of the laboratory.
Results
Pre-conversion
Caseload and FTE
In May 2019, prior to the conversion, Dr. Joaquim Chaves Saúde Clinical Analysis Laboratory (JCS) processed on average 492 samples/day on weekdays, 389 on Saturdays, and approximately 12 on Sundays. Three-quarters of the samples received were urine samples, 16% were vaginal swabs, 4% stool samples, 2% pharyngeal swabs, and about 1% were samples sent in for mycology testing. Other sample types comprised 3%. The laboratory had 10 FTEs of staff at the time.
KPIs
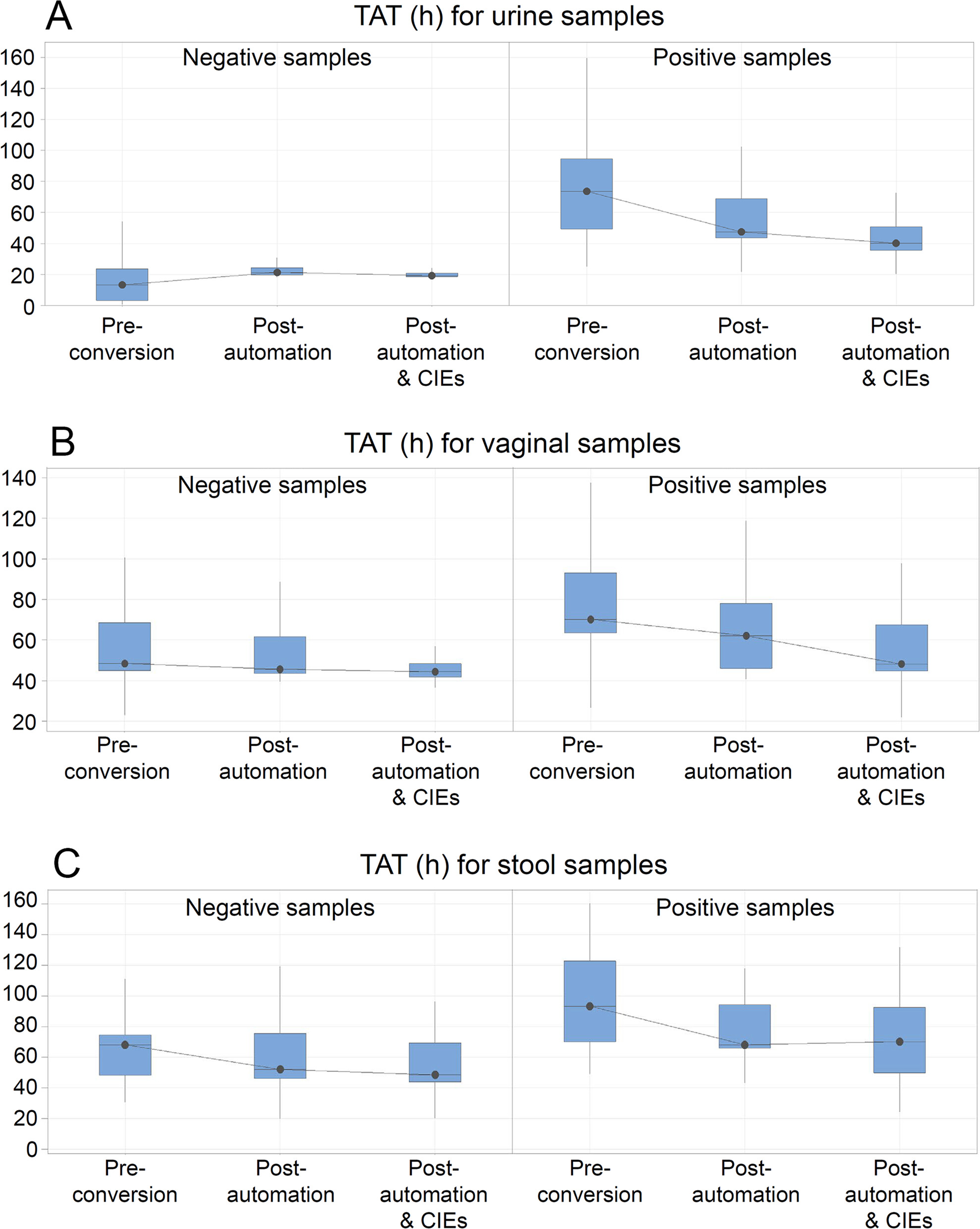
The productivity pre-conversion was 49 samples/FTE/day (Table 1). The median TAT was the shortest for the negative urine samples (median 13 hours; interquartile range [IQR; 3.1; 23.7]) and the longest for the positive stool samples (median 93.3 hours; IQR [70.1; 122.8]). Positive urine and vaginal swabs, in comparison, had a similar median TAT with a narrower IQR for vaginal swabs (median 73.7 hours; IQR [43.4; 94.6] versus median 70.3 hours; IQR [64.5; 93.1]). Full results are given in Table 2.
| ||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Post-automation
Caseload and FTE
In March 2021, following the introduction of automation, the average number of samples in a day processed by JCS equaled 621 on weekdays, 417 on Saturdays, and about11 on Sundays. The distribution of sample types changed only slightly. Seventy-eight percent of samples received were urine samples, 12% were vaginal swabs, 2% were stools, and 1% were pharyngeal swabs, and approximately 1% were samples sent in for mycology testing. Other sample types comprised 6%. In March 2021, the availability of FTEs in the laboratory went down to 8.5 (Table 1).
KPIs
Given the average number of samples received in a day and the reduced FTEs, the productivity of the JCS laboratory increased to 73 samples/FTE/day (Table 1). The automation led to a decrease in TAT for all sample types except for negative urine samples due to the discontinuation of the UF1000i urinalysis system, whereas the decrease by 26% of the TAT for positive stool samples did not reach statistical significance (P = 0.121). Nonetheless, the median TAT was still the shortest for the negative urine samples (median 21.3 hours; IQR [21.2; 22.9]) and the longest for the positive stool samples (median 69.5 hours; IQR [67.3; 94.4]). It is important to mention that for the urine workflow, the removal of the screening step using the UF1000 explains the longer TAT for negative urine, since currently, the sample status depends on the interpretation of culture on agar. TAT for positive urine samples went down to a median of 47.4 hours (43.5; 68.7), while positive vaginal swabs decreased to a median of 62.2 hours (46.1; 85.1) (Table 2).
Post-automation and CIEs
Caseload and FTE
In September 2021, the average number of samples processed and the available FTEs remained unchanged since March 2021.
KPIs
Automation, together with continuous improvement events, increased productivity to a theoretical level of 110/FTE/day, equivalent to processing 935 samples/day with the same FTEs, which more than doubled the original productivity observed pre-conversion (Table 1). Further improvement of median TAT was observed for positive urine (median 40.0 hours; IQR [35.6; 50.7]) and vaginal swab (median 48.2 hours; IQR [44.8; 67.7]) samples, while no additional improvement was seen for the positive stool samples. Predefined goals were reached for all samples except for positive stool specimens (Table 2).
The introduction of WASPLab and VITEK MS together with the CIEs led to a reduction in variability of TAT, shown as a decrease in the IQR range (Table 2). For the negative samples, an IQR reduction of 89%, 72%, and 3% was observed for urine, vaginal swab, and stool specimens, respectively, while the values equaled 67%, 23%, and 19% for the positive counterparts.
The relative impact of automation and CIEs on overall reduction in TAT
Laboratory conversion (automation and CIEs) led to a 124% improvement in productivity and improved TATs by a value ranging from 8% (for negative vaginal swabs) to 46% (for positive urine samples). TAT for positive vaginal swabs and stool samples improved by 31% and 26%, respectively (Table 2).
Discussion
The conversion of the JCS laboratory, consisting of laboratory automation facilitated by change management, substantially improved the productivity and TATs of the laboratory and reached goals predefined by the management. The improvement was the greatest for the positive urine samples, for which the median TAT decreased from just over three days (73.7 hours) to well under two days (40 hours, Fig. 1). The discontinuation of the UF1000 automated urine particle analyzer caused the expected lengthening of TAT for negative urine samples, although the post-conversion median TAT met the expected goal predefined by the JCS management. Moreover, TAT variability decreased following laboratory automation and CIEs. This occurred through an improvement of the standardization of the processes and full alignment with the pre-established priorities.
|
A more streamlined automatic workflow that eliminated many manual tasks provided the major contribution to TAT reduction; however, CIEs maximized the potential benefit of laboratory automation and contributed substantially to productivity improvements. In 2019, the lab was processing, on average, 492 samples a day with 10 FTEs; after laboratory automation, the lab was processing 621 samples a day with 8.5 FTEs. After CIEs, with 8.5 FTEs, the lab could process 935 samples per day. In 2019, 19 FTEs would have been needed to process a daily caseload of 935 specimens, whereas after the integration of laboratory automation, 13 FTEs would have been needed. In other words, had there been a caseload of 935 at the time, the improvement in productivity observed following lab conversion and optimization of workflows would have allowed for the saving of six FTEs attributable to laboratory automation alone, and of an additional 4.5 FTEs made possible by the application of lean principles (i.e., the CIEs).
The delivery of the change management program in JCS helped the laboratory staff to increase their level of understanding of automation. The change management program focused on defusing reluctance to technological changes, motivated the adoption of WASP and WASPLab technologies, developed confidence toward hardware and software systems, envisioned daily work with automation, worked on the understanding that skills are transformed and not lost, and clarified the expectations and decisions to be made within the laboratory. The procedure optimization phase based on lean management application allowed the team of the Department of Microbiology at the JCS to develop a mindset of continuous improvement that is needed to maximize the potential of laboratory automation, reach preset goals, and to sustain them, which is a fundamental element of lean management. [14]
The results of this study confirm the findings published by others. In the setting of a microbiology laboratory, laboratory automation has historically been shown to improve efficiency. [15–22] In addition, the combination of matrix-assisted laser desorption/ionization–time-of-flight mass spectrometry (MALDI-TOF)-based microbial identification and total laboratory automation individually and together improved TATs in microbiology. [23, 24] Da Rin and colleagues showed that the integration of the WASPLab diagnostic microbiology system into a pre-existing system of total automation, together with resource standardization and optimization, shortened TAT. [25] In a Chinese study, total laboratory automation was accompanied by the setting up of three working shifts, as opposed to a single working shift pre-automation. Together, the two actions led to a statistically significant shortening of TATs, especially for the cerebrospinal fluid sample diagnostics. [26]
Unlike Cherkaoui and colleagues, who saw only a trend toward shorter median TATs, our intervention led to a substantial improvement in TAT for positive urine samples. This result strongly supports the notion that automation must be matched by laboratory work organization, which was a limitation acknowledged by Cherkaoui et al. in their study. [27] In our study, laboratory automation alone was responsible for 37.2% and 75.8% reductions in TAT observed for positive vaginal swabs and positive urine samples, respectively, while the remaining 62.8% and 24.2% reduction in TAT for these samples was achieved due to CIEs. The study by Yarborough et al. confirmed that changes in urine sample workflow were necessary to maximize the efficiency of laboratory automation and to optimize TAT. [28]
Taken together, the conversion of the JCS laboratory resulted in several benefits. The first benefit was the occurrence of fewer errors and associated rework. Most of the laboratory errors occur during the extra (pre- and post-) analytical phases of the testing process. [29] Indeed, in the workflow before the conversion, there was a lot of room for errors since some of the samples were labeled manually and test results were manually fed into the laboratory information system (LIS). Secondly, the conversion led to fewer uncertainties concerning operating procedures, which in turn counteracted the feeling of being overwhelmed, a sensation that may be experienced by laboratory staff after a major change to the working routine. Thirdly, during the conversion, an environment favorable to continuous improvement was created, with clear procedures allowing any problem or deviation to be easily spotted. In addition, sample type-based CIEs enabled focused collaboration of the technologists and sequential validation. Such sample type-based optimization was also done by Cherkaoui et al. in Geneva. [30] Lastly, productivity improvements facilitated qualified staff transfer to clinical activities despite working in the context of the COVID-19 pandemic, which led to a 20% caseload increase.
At the level of global trends, the introduction of laboratory automation and CIE helps counteract problems with staffing experienced by most of the clinical laboratories worldwide due to accelerated rates of microbiologists’ retirement and fewer people entering the field of laboratory medicine. A very recent study showed that up to 80% of microbiology laboratories have vacancies and struggle to fill them. [31] There is also the phenomenon of declining reimbursement, and total laboratory automation is a way of obtaining cost savings. [32]
There is one main limitation to the study, which is that it is the experience of one center. While laboratory automation has been demonstrated to provide benefits regardless of site size/organization [4], the magnitude of the benefit and impact of CIEs are likely to be site-dependent since they require finding the right combination of people, processes (i.e., workflow), and technology to unlock new sources of improvement. [11] Moreover, the productivity index used in the study is strongly linked to the relative local distribution of sample types, which at the JCS laboratory was 74% urine samples, 16% vaginal swabs, 4% stool samples, and 6% other specimens. Consequently, the improvements in productivity observed after lab automation and after CIEs would be expected to vary with a different sample distribution.
A minor limitation of the study is the fact that the workflow for positive stool samples did not reach the predefined TAT goal of 65 hours. However, stool samples represent only 4% of the caseload, and only 5% of them are positive, which makes the standardization of the workflow for these isolated specimens difficult. Furthermore, while previous studies by Cherkaoui and colleagues also highlighted the potential of automation to reduce TAT for other critical samples such as blood culture [33], the caseload for this sample type in the JCS laboratory was too low to be included in the present study. Moreover, the present study looked at the impact of a bundled intervention, i.e., implementation of WASPLab and VITEK MS systems. While the decrease of TAT is most likely caused by the combination of both technologies, the relative impact of each of the changes was not assessed.
Conclusion
In conclusion, JCS conversion resulted in substantial improvements in KPIs. While automation alone substantially improved TAT and productivity, the subsequent implementation of lean management further unlocked the potential of laboratory automation through the streamlining of the processes involved. Together, they led to the achievement of predefined goals. Future studies will be needed to see how additional developments in the field of automation—e.g., automatic colony picking for identification by MALDI-TOF [34] and antimicrobial susceptibility, as well as novel algorithms for automated reading and interpretation of plates [6–8]—could further improve productivity and the quality of results.
Materials and methods
The project
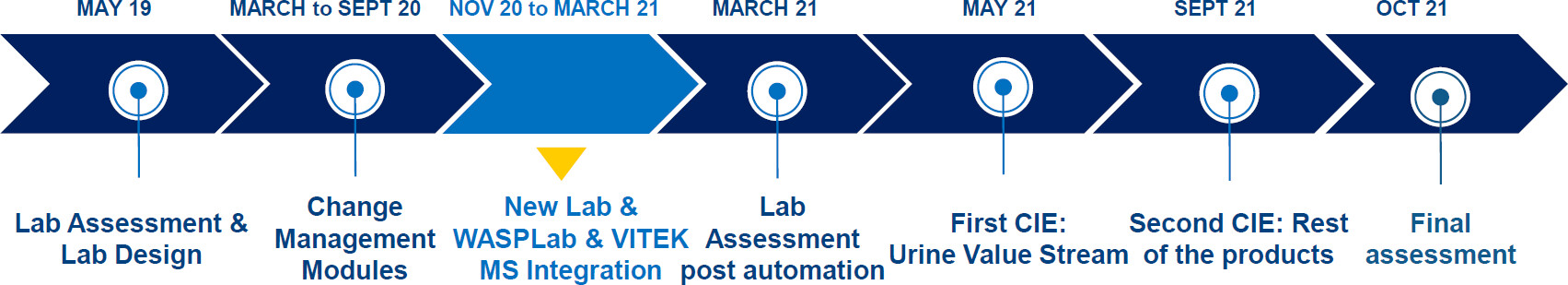
The project took place at the JCS in Lisbon, Portugal between May 2019 and October 2021. In 2019, a full assessment of the laboratory workflow and performance was made to size the instruments’ needs and to propose a lean design for the future microbiology laboratory, considering the different types of workflows of the laboratory. A decision was made to automate the laboratory and remove the UF1000 automated system for urine particle analysis (Sysmex, Japan). The latter move was dictated by the desire to simplify and streamline the processing of this specimen type.
In November 2020, the laboratory moved to the new facilities, where WASPLab (Copan Diagnostics, Inc.) for automated specimen processing and reading and VITEK MS (bioMérieux, France), a MALDI-TOF-based microbial identification system, were installed. For urine samples, a steady routine state was achieved by March 2021. For stool and vaginal swab samples, such a routine was reached by May 2021.
In May 2021, the first CIE event took place and concentrated on the urine workflow. Four months later, in September 2021, a second CIE was conducted for stool and vaginal swab samples. The final assessment of the KPIs was performed in October 2021. The chronology of the project is shown in Fig. 2. Across the three time points, the laboratory processed samples during similar opening times from 8 a.m. to 10 p.m. in two shifts.
|
Acknowledgements
Funding
Competing interests
Yohann Bala, Monica Gutiérrez Granado, Sandra Vallejo, and Victoria Gonzalez are employees of bioMérieux SA.
References
Notes
This presentation is faithful to the original, with only a few minor changes to presentation. In some cases important information was missing from the references, and that information was added.