Featured article of the week: April 6–12:
Health informatics (also called health care informatics, healthcare informatics, medical informatics, nursing informatics, clinical informatics, or biomedical informatics) is a discipline at the intersection of information science, computer science, and health care. It deals with the resources, devices, and methods required to optimize the "collection, storage, retrieval, [and] communication ... of health-related data, information, and knowledge." Health informatics is applied to the areas of nursing, clinical care, dentistry, pharmacy, public health, occupational therapy, and biomedical research. Health informatics resources include not only computers but also clinical guidelines, formal medical terminologies, and information and communication systems.
Worldwide use of technology in medicine began in the early 1950s with the rise of computers. Medical informatics research units began to appear during the 1970s in Poland and in the U.S., with medical informatics conferences springing up as early as 1974. Since then the development of high-quality health informatics research, education, and infrastructure has been the goal of the U.S. and the European Union. Hundreds of datasets, publications, guidelines, specifications, meetings, conferences, and organizations around the world continue to shape what health informatics is today. (Full article...)
Featured article of the week: March 30–April 5:
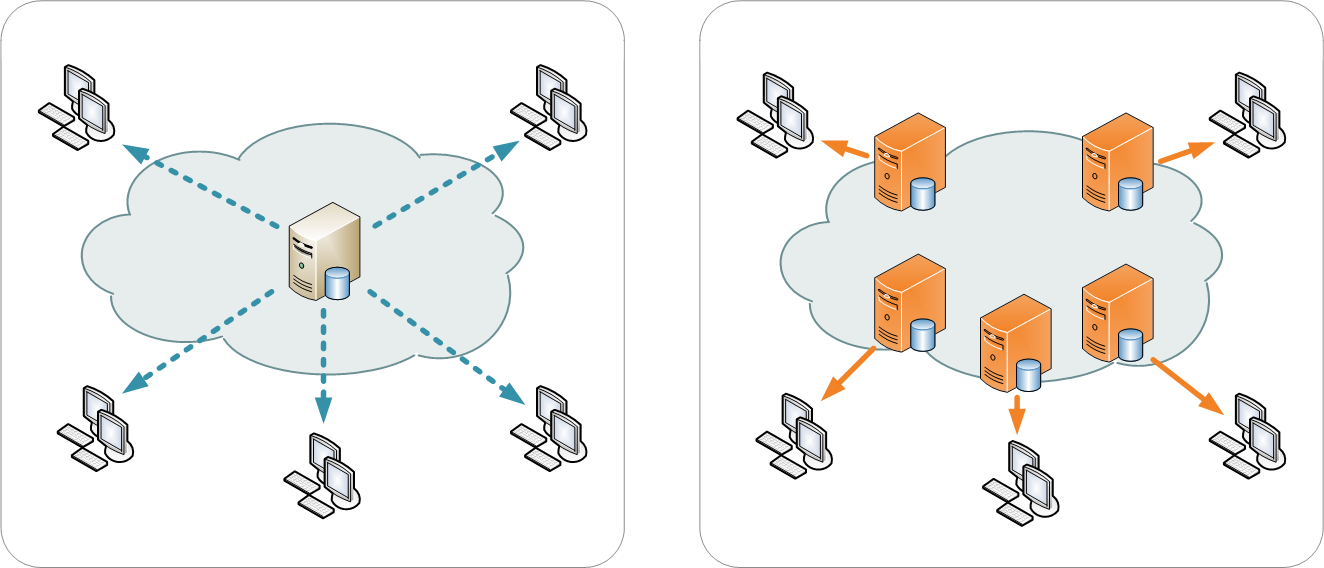
A content delivery network or content distribution network (CDN) is a large distributed system of servers deployed in multiple data centers or "nodes" across the Internet. The goal of a CDN is to serve content to end-users with the intended benefit of reducing bandwidth costs, improving page load times, and/or increasing global availability of content. This is done by hosting the content on several servers, and when a user makes a request to CDN-hosted content, the domain name server (DNS) will resolve to an optimized server based on location, availability, cost, and other metrics.
Content providers such as media companies and e-commerce vendors pay CDN operators to deliver their content to their audience of end-users. In turn, a CDN pays Internet service providers (ISPs), carriers, and network operators for hosting its servers in their data centers. Besides better performance and availability, CDNs also offload the traffic served directly from the content provider's origin infrastructure, resulting in possible cost savings for the content provider. In addition, CDNs provide the content provider a degree of protection from denial-of-service (DoS) attacks by using their large distributed server infrastructure to absorb the traffic.
An increasing number of ISPs have built their own CDNs to improve on-net content delivery, reduce demand on their own infrastructure, and generate revenues from content customers. Additionally, some companies such as Microsoft, Amazon, and Netflix have built their own CDNs to tie in with their own products. (Full article...)
|
Featured article of the week: March 23–29:
A federally qualified health center (FQHC) is a reimbursement designation from the Centers for Medicare and Medicaid Services (CMS) of the United States Department of Health and Human Services (HHS). This designation is significant for several health programs funded under Section 330 of the Public Health Service Act, as part of the Health Center Consolidation Act. The FQHC program is designed "to enhance the provision of primary care services in underserved urban and rural communities."
FQHCs are community-based organizations that provide comprehensive primary and preventive care, including health, oral, and mental health services to persons of all ages, regardless of their ability to pay or health insurance status. Thus, they are a critical component of the health care safety net. As of 2011 over 1,100 FQHCs operate approximately 6,000 sites throughout the United States and territories, serving an estimated 20 million patients. That number is expected to go up to 40 million people by 2015 thanks to extra grant funding to the program. FQHCs may also be referred to as community/migrant health centers (C/MHC), community health centers (CHC), and 330 funded clinics. (Full article...)
|
Featured article of the week: March 16–22:
A home health agency (HHA) is a public agency, private organization, or a subdivision of such dedicated to providing health care services to people in their residence or in another non-institutional setting. Care may be provided by licensed healthcare professionals who provide medical care needs or by professional caregivers who provide daily care to help to ensure the activities of daily living (ADL's) are met. Often, the term "home health care" is used to distinguish a home health agency's services from personal, non-medical, custodial, or private-duty care services, which are provided by persons who are not nurses, doctors, or other licensed medical personnel.
In 2010, over 10,800 Medicare-certified home health agencies operated throughout the United States, serving 3,446,057 beneficiaries over 122,578,603 visits. Services at home health agencies vary widely. Common categories of services include taking and recording vital signs, turning and positioning bed-bound patients, assisting in the self-administration of medication, conducting physical and occupational therapy, and providing medical social work, among other activities. (Full article...)
|
Featured article of the week: March 9–15:
ISO 9000 is a family of standards related to quality management systems and designed to help organizations ensure that they meet the needs of customers and other stakeholders. The standards are published by the International Organization for Standardization (ISO) and are available through national standards bodies. ISO 9000 deals with the fundamentals of quality management systems, including the eight management principles on which the family of standards is based.
The global adoption of the ISO 9000 family of standards may be attributable to a number of factors. Many major purchasers require their suppliers to hold ISO 9001 certification. In addition to several stakeholders' benefits, a number of studies have identified significant financial benefits for organizations certified to ISO 9001. Over a million organizations worldwide are independently certified, making ISO 9001 one of the most widely used management tools in the world today. Despite widespread use, however, the ISO certification process has been criticized as being wasteful and not being useful for all organizations. (Full article...)
|
Featured article of the week: March 2–8:
Health Level Seven (HL7) is an international non-profit volunteer-based organization involved with the development of international health care informatics interoperability standards. The HL7 community consists of health care experts and information scientists collaborating to create standards for the exchange, management, and integration of electronic health care information. The term "HL7" is also used to refer to some of the specific standards created by the organization (e.g., HL7 v2.x, v3.0, HL7 RIM). HL7 and its members provide a framework (and related standards) for the exchange, integration, sharing, and retrieval of electronic health information. v2.x of the standards, which support clinical practice and the management, delivery, and evaluation of health services, are the most commonly used in the world.
In total HL7 develops conceptual standards (e.g., HL7 RIM), document standards (e.g., HL7 CDA), application standards (e.g., HL7 CCOW), and messaging standards (e.g., HL7 v2.x and v3.0). The HL7 messaging standards v2.x and 3.0 are the primary standards from the organization. They provide a framework for data exchange among clinical and healthcare systems in an ideal format. The 2.x standards are flexible, with several implementation options, loosely geared towards "clinical interface specialists" working to move clinical data in the application space. The 3.0 standards are designed to be more fixed, precise, and international, geared towards governments and end users of clinical applications. (Full article...)
|
Featured article of the week: February 22–March 1:
ISO/IEC 17025 is an International Organization for Standardization (ISO) standard used by testing and calibration laboratories to provide a basis for accreditation of laboratory quality systems. There are many commonalities with the ISO 9000 family of standards, but ISO/IEC 17025 adds in the concept of competence to the equation, applying directly to those organizations that produce testing and calibration results.
ISO/IEC 17025:1999 was issued by the ISO in late 1999 and was internationally adopted in 2000. A second release was made on May 12, 2005 after it was agreed that it needed to have its wording more closely aligned with the 2000 version of ISO 9001. The most significant changes introduced greater emphasis on the responsibilities of senior management, as well as explicit requirements for continual improvement of the management system itself, particularly communication with the customer.
The ISO/IEC 17025 standard itself comprises five elements: scope, normative references, terms and definitions, management requirements, and technical requirements. In particular the management and technical requirements are the most important sections, with the management requirement section detailing the operation and effectiveness of the quality management system within the laboratory and the technical requirements section detailing the factors which determine the correctness and reliability of the tests and calibrations performed in laboratory. (Full article...)
|
Featured article of the week: February 16–22:
Good Automated Manufacturing Practice (GAMP) is both a technical subcommittee of the International Society for Pharmaceutical Engineering (ISPE) and a set of guidelines for manufacturers and users of automated systems in the pharmaceutical industry. One of the core principles of GAMP is that quality cannot be tested into a batch of product but must be built into each stage of the manufacturing process. As a result, GAMP covers all aspects of production; from the raw materials, facility and equipment to the training and hygiene of staff.
GAMP is largely about automated system validation. In October 2014, Irish tech company Dataworks Ltd. described it as such:
"It is a formal process of thorough documentation, testing, and logical process steps that validate clients' required specifications. The process begins with a user requirements specification for the machine, from which a functional requirement and a design specification are created. These documents then form the basis for the traceability matrix and for the formal testing of internal acceptance, factory acceptance, and site acceptance. Categorising software is used to support the approach to validation based on the difficulty and individuality of the computerised system." (Full article...)
|
Featured article of the week: February 9–15:
An end-stage renal disease facility (ESRD facility, dialysis facility, or dialysis center) is a medical facility that operates to assist people with irreversible loss of kidney function (stage five), requiring a regular course of dialysis or a kidney transplant to survive. The facility may operate independently, as part of a hospital-based unit, or as a self-care unit that furnishes only self-dialysis services.
The U.S. Centers for Medicare and Medicaid Services (CMS) describes four types of ESRD facilities, including the renal transplantation center, for ESRD transplant patients; the renal dialysis center, a hospital unit for ESRD dialysis patients; a renal dialysis facility, a direct or stand-alone dialysis unit for ESRD patients; and a self-dialysis unit attached to one of the previous three, providing self-dialysis services. Patients undergoing dialysis at these facilities require two important documentation steps: the patient assessment and the patient plan of care. U.S. Federal regulation requires a comprehensive 13-point assessment, including current health status, laboratory profile, and nutritional status. (Full article...)
|
Featured article of the week: February 2–8:
Genome informatics is a field of computational molecular biology and branch of informatics that uses computers, software, and computational solution techniques to make observations, resolve problems, and manage data related to the genomic function of DNA sequences, comparison of gene structures, determination of the tertiary structure of all proteins, and other molecular biological activities. The informatics side of genomics has largely focused on analytical tools and methodologies. DNA-microarray and sequencing technology helped researchers for the Human Genome Project, for example, analyze and understand thousands of genes and their expressions. By 2000, artificial neural networks were being theorized as a possible informatics tools to aid with data analysis and the problem of "high dimensionality" of the outputted data; by 2014 artificial neural networks were being proposed for cancer genomic research.
Genome informatics can help tackle problems and tasks such as analyzing DNA sequences, recognizing genes and proteins and predicting their structures, and predicting the biochemical function of new genes or fragments, as well as molecular profiling. (Full article...)
|
Featured article of the week: January 26–February 1:
Cancer informatics is a multidisciplinary field of science that "deals with the resources, devices, and methods required to optimize the acquisition, storage, retrieval, and use of information in cancer" research and treatment. Like many other fields of science, researchers in cancer biology have seen a dramatic increase in the amount of clinical and research data, in particular with genomic and molecular cancer data. While this data can benefit researchers' understanding of cancer behavior and development of better therapies, new and improved data management and analysis tools are needed. Cancer informatics attempts to provide those tools "that interconnect research, clinical activities, and data in an organized and efficient manner, with as broad a database as possible." For many, the coupling of cancer informatics and other bioinformatics tools with computational modeling and statistical analysis will accelerate the goal of making cancer a more treatable if not curable disease.
Cancer informatics can help tackle problems and tasks such as the development of computational diagnosis, prognosis, and predictive models; the development of standards for the entry, annotation, and sharing of clinical cancer data; and the management and distribution of annotated molecular data for further research. (Full article...)
|
Featured article of the week: January 19–25:
Evolutionary informatics is a sub-branch of informatics that addresses the algorithmic and technological tools (like information and analytical systems) needed to better manage data from research in ecology and evolutionary biology and answer evolutionary questions. As in bioinformatics and genomics, scientists studying biological evolution have gathered an increasingly large volume of information, resulting in information management problems. Additionally, as bioinformatics and genomics are pertinent to the study of evolution, utilization of information from those areas is of concern in evolutionary informatics.
Evolutionary informatics has evolved out of a wide variety of scientific, mathematical, and computational endeavors, including evolutionary biology, evolutionary computation, algorithmic and evolutionary algorithmic research, and software development. It can help tackle problems and tasks such as reducing "the growing number of lineages that lack formal taxonomic names," digitizing and semantically enhancing legacy biodiversity data while also making it more portable, and building "sustainable digital community repositories that provide access to rich data and metadata" in the field of evolutionary biology. (Full article...)
|
Featured article of the week: January 12–18:
A scientific data management system (SDMS) is a piece or package of software that acts as a document management system (DMS), capturing, cataloging, and archiving data generated by laboratory instruments (HPLC, mass spectrometry) and applications (LIMS, analytical applications, electronic laboratory notebooks) in a compliant manner. The SDMS also acts as a gatekeeper, serving platform-independent data to informatics applications and/or other consumers.
As with many other laboratory informatics tools, the lines between a LIMS, ELN, and an SDMS are at times blurred. However, there are some essential qualities that an SDMS owns that distinguishes it from other informatics systems. It's built to handle unstructured, mostly heterogeneous data; it typically acts as a seamless "wrapper" for other data systems like LIMS and ELN in the laboratory; and it is designed primarily for data consolidation, knowledge management, and knowledge asset realization. An SDMS also must be focused on increasing research productivity without sacrificing data sharing and collaboration efforts. (Full article...)
|
Featured article of the week: January 5–11:
 The Centers for Disease Control and Prevention (CDC) is the national public health institute of the United States. The CDC is a federal agency under the Department of Health and Human Services and is headquartered in Atlanta, Georgia. Its main goal is to protect public health and safety through the control and prevention of disease, injury, and disability. The CDC focuses national attention on developing and applying disease control and prevention. It especially focuses its attention on infectious disease, food borne pathogens, environmental health, occupational safety and health, health promotion, injury prevention, and educational activities designed to improve the health of United States citizens. The CDC combats emerging diseases and other health risks, including birth defects, West Nile virus, avian influenza, swine influenza, pandemic flu, E. coli, and bioterrorism, to name a few. In addition, the CDC researches and provides information on non-infectious diseases such as obesity and diabetes and is a founding member of the International Association of National Public Health Institutes.
The CDC has one of the few biosafety level 4 laboratories in the country, as well as one of only two official repositories of smallpox in the world. The second smallpox store resides at the State Research Center of Virology and Biotechnology VECTOR in the Russian Federation. (Full article...)
|
Featured article of the week: December 29–January 4:
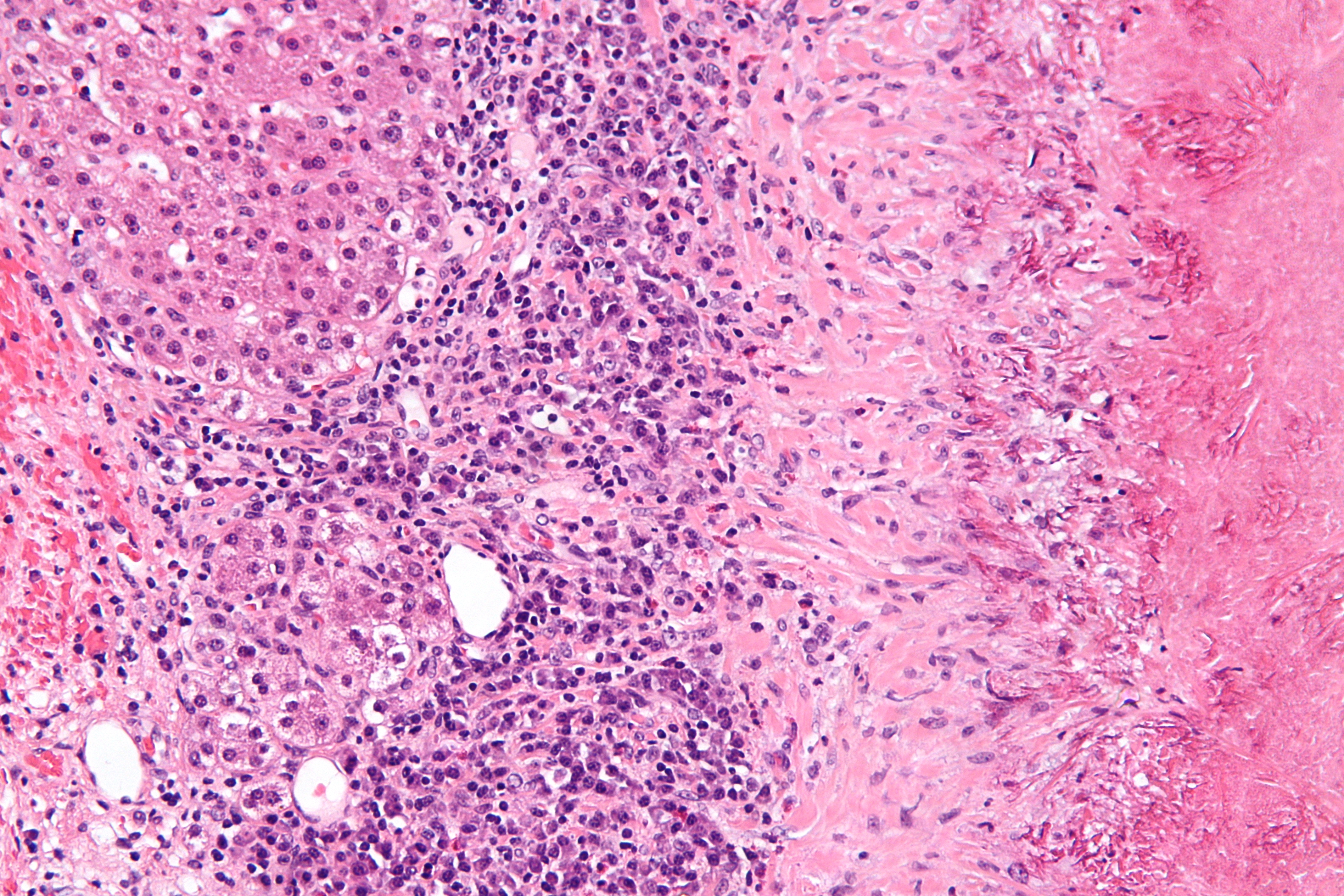
Histopathology is a branch of histology and pathology that studies and diagnoses diseases on the tissue and cellular level. While histopathology is closely related to cytopathology, the main difference is diagnostic information gained from histopathology is acquired from solid tissue samples, whereas specific disaggregated cell preparations are used in cytopathology. Typically a biopsy or surgical specimen is examined by a pathologist after the specimen has been processed and histological sections have been placed onto glass slides. Testing typically incorporates several stages, from collection and preparation using numerous methods (depending on the sample and test type) down to examination.
Rudolf Ludwig Karl Virchow is considered by many to be one of the fathers of cellular pathology, remembered most for his collection of lectures on the topic, published as Cellular Pathology in 1858. However, his assistant, David Paul von Hansemann also played an important role in the progress of histopathology during the 1890s, producing his book The Microscopic Diagnosis of Malignant Tumours and other important research. (Full article...)
|
|