Difference between revisions of "Main Page/Featured article of the week/2022"
Shawndouglas (talk | contribs) (Created as needed.) |
Shawndouglas (talk | contribs) (Added last week's article of the week) |
||
| Line 23: | Line 23: | ||
Accessible [[Epidemiology|epidemiological]] data are of great value for emergency preparedness and response, understanding disease progression through a population, and building statistical and mechanistic disease models that enable forecasting. The status quo, however, renders acquiring and using such data difficult in practice. In many cases, a primary way of obtaining epidemiological data is through the internet, but the methods by which the data are presented to the public often differ drastically among institutions. As a result, there is a strong need for better data sharing practices. This paper identifies, in detail and with examples, the three key challenges one encounters when attempting to acquire and use epidemiological data: (1) interfaces, (2) data formatting, and (3) reporting. ('''[[Journal:Epidemiological data challenges: Planning for a more robust future through data standards|Full article...]]''')<br /> | Accessible [[Epidemiology|epidemiological]] data are of great value for emergency preparedness and response, understanding disease progression through a population, and building statistical and mechanistic disease models that enable forecasting. The status quo, however, renders acquiring and using such data difficult in practice. In many cases, a primary way of obtaining epidemiological data is through the internet, but the methods by which the data are presented to the public often differ drastically among institutions. As a result, there is a strong need for better data sharing practices. This paper identifies, in detail and with examples, the three key challenges one encounters when attempting to acquire and use epidemiological data: (1) interfaces, (2) data formatting, and (3) reporting. ('''[[Journal:Epidemiological data challenges: Planning for a more robust future through data standards|Full article...]]''')<br /> | ||
|- | |- | ||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: | |<br />//--><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: January 3–9:</h2> | ||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File: | <div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig8 Lee Sustain20 13-1.png|240px]]</div> | ||
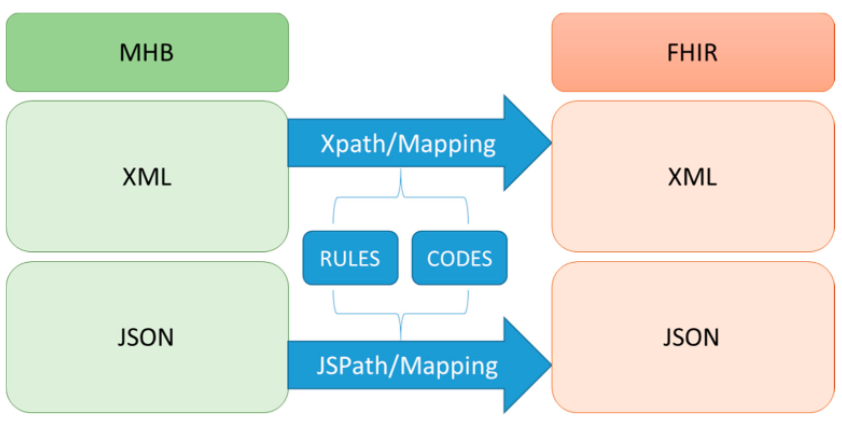
'''"[[Journal: | '''"[[Journal:Implement an international interoperable PHR by FHIR: A Taiwan innovative application|Implement an international interoperable PHR by FHIR: A Taiwan innovative application]]"''' | ||
[[ | [[Personal health record]]s (PHRs) have many benefits for things such as [[Public health surveillance|health surveillance]], [[Epidemiology|epidemiological surveillance]], self-control, links to various services, [[public health]] and health management, and international surveillance. The implementation of an international standard for interoperability is essential to accessing PHRs. In Taiwan, the nationwide exchange platform for [[electronic medical record]]s (EMRs) has been in use for many years. The [[Health Level 7|Health Level Seven International]] (HL7) Clinical Document Architecture (CDA) was used as the standard for those EMRs. However, the complication of implementing CDA became a barrier for many [[hospital]]s to realizing standard EMRs. In this study, we implemented a [[Health Level 7#Fast Healthcare Interoperability Resources (FHIR)|Fast Healthcare Interoperability Resources]] (FHIR)-based PHR transformation process, including a user interface module to review the contents of PHRs. ('''[[Journal:Implement an international interoperable PHR by FHIR: A Taiwan innovative application|Full article...]]''')<br /> | ||
|- | |- | ||
|} | |} | ||
|} | |} | ||
<br /> | <br /> | ||
<div align="center">[[Main Page|— Return to the front page —]]</div> | <div align="center">[[Main Page|— Return to the front page —]]</div> | ||
Revision as of 17:09, 10 January 2022
|
|
If you're looking for other "Article of the Week" archives: 2014 - 2015 - 2016 - 2017 - 2018 - 2019 - 2020 - 2021 - 2022 |
Featured article of the week archive - 2022
Welcome to the LIMSwiki 2022 archive for the Featured Article of the Week.
Featured article of the week: January 3–9:"Implement an international interoperable PHR by FHIR: A Taiwan innovative application" Personal health records (PHRs) have many benefits for things such as health surveillance, epidemiological surveillance, self-control, links to various services, public health and health management, and international surveillance. The implementation of an international standard for interoperability is essential to accessing PHRs. In Taiwan, the nationwide exchange platform for electronic medical records (EMRs) has been in use for many years. The Health Level Seven International (HL7) Clinical Document Architecture (CDA) was used as the standard for those EMRs. However, the complication of implementing CDA became a barrier for many hospitals to realizing standard EMRs. In this study, we implemented a Fast Healthcare Interoperability Resources (FHIR)-based PHR transformation process, including a user interface module to review the contents of PHRs. (Full article...) |