Difference between revisions of "Journal:Laboratory information management system for COVID-19 non-clinical efficacy trial data"
Shawndouglas (talk | contribs) (Saving and adding more.) |
Shawndouglas (talk | contribs) (Saving and adding more.) |
||
| Line 45: | Line 45: | ||
==Results== | ==Results== | ||
===User management and functionalities=== | ===User management and functionalities=== | ||
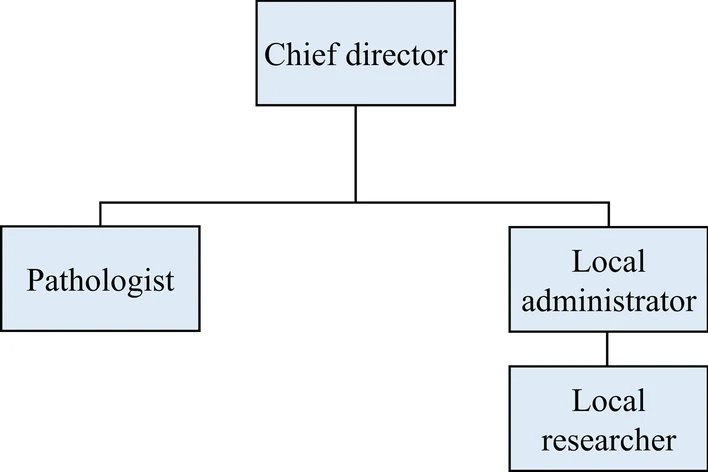
Our COVID-19 non-clinical trial LIMS has four different types of users with different authorizations to handle multiple organizations: a chief director, a local administrator and researcher from each organization, and a [[Pathology|pathologist]] (Fig. 1). The role of the chief director includes new project initiation, modification, deactivation, user account management, and data management. The chief director can also control the registration of new organizations and the removal of existing organizations. The role of the local administrators is the same as that of the chief director, except that they are limited to the organization to which they belong. This enables the efficient management of each organization, as well as [[Cybersecurity|data security]]. Researchers can manage data entry and removal and check the project's progress. Pathologists can conduct pathological analyses, pathological data entry, and related functions. Furthermore, multiple user types for multiple roles are allowed. If multiple authorizations are granted, the screen manual is shown based on the user’s choice. | |||
[[File:Fig1 Yoon LabAniRes22 38.png|500px]] | |||
{{clear}} | |||
{| | |||
| style="vertical-align:top;" | | |||
{| border="0" cellpadding="5" cellspacing="0" width="500px" | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |<blockquote>'''Figure 1.''' Authority organization chart. The proposed LIMS fully supports cooperative multi-center study. In this respect, each institute can have pathologists and multiple local researchers and administrators, directed by a chief director.</blockquote> | |||
|- | |||
|} | |||
|} | |||
The proposed system, which applies a convenient user interface, was created to provide many functions related to [[Information management|data management]] for multi-center research. The detailed function list is presented in Table 1. | |||
{| | |||
| style="vertical-align:top;" | | |||
{| class="wikitable" border="1" cellpadding="5" cellspacing="0" width="80%" | |||
|- | |||
| colspan="3" style="background-color:white; padding-left:10px; padding-right:10px;" |'''Table 1.''' Detailed function list of the LIMS.<br /> <br /><sup>C</sup>: Creation; <sup>U</sup>: Update; <sup>A</sup>: Activation; <sup>D</sup>: Deactivation; <sup>+</sup>: Administrator-only function; <sup>*</sup>: Principal investigator-only function; <sup>†</sup>: Pathologist-only function; <sup>※</sup>: Notified by e-mail or social messenger upon action | |||
|- | |||
! style="padding-left:10px; padding-right:10px;" |Category | |||
! style="padding-left:10px; padding-right:10px;" |Function | |||
! style="padding-left:10px; padding-right:10px;" |Note | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" rowspan="6"|Access management | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |<sup>+</sup> Management (C/U/D) | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" | | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |<sup>※</sup> Join member | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" | | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |<sup>※</sup><sup>+</sup><sup>*</sup> Grant member | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" | | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Sign-in/out | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" | | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Confirm EULA | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Appeared once | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Manuals (system, injection demo, autopsy protocol/checklist/demo) | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" | | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" rowspan="13"|Trial | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |<sup>※</sup><sup>+</sup> Initialize trial | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" | | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |<sup>※</sup><sup>*</sup> Activate trial | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" | | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Participation confirmation | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Appeared once | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |<sup>U</sup> Data management (run trial) | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" | | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Visualize current data | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" | | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |<sup>+</sup><sup>*</sup> Export trial data | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" | | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |<sup>A</sup><sup>D</sup> Additional dataset management | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" | | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |<sup>※</sup><sup>*</sup> Request pathological analysis | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" | | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |<sup>†</sup> Perform pathological analysis | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" | | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |<sup>※</sup><sup>†</sup> Finish pathological analysis | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" | | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |<sup>*</sup> Rollout trial report | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" | | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |<sup>※</sup><sup>*</sup> Request trial completion | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" | | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |<sup>※</sup><sup>+</sup> Complete trial | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" | | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" rowspan="3"|Audit | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Activity logging | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Automatic | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |<sup>+</sup> Activity review and search (by trial, date, sample, or member) | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" | | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |<sup>+</sup> Revert or recover the data management activity | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" | | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" rowspan="2"|Comparison | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Select trial (by type, objective, institution, model, data, institution) | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" | | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Visualize the selected trials (by type, objective, model, institution) | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" | | |||
|- | |||
|} | |||
|} | |||
Functions can be classified into four different categories: access management, trial, audit, and comparison. The access management function forms the basis of the system and manages the user group. The trial function controls the entire project process, including initiation, progression, and finalization. The [[Audit trail|audit function]] records all activities that occur in the system and implements data entry, cancelation, and restoration. The comparison function provides several plots according to the investigational product (new drug/repositioning, vaccine/therapeutic), sponsor, experimental animals (hamster model/hACE2 mouse model), research objective, and study site. It performs access control and visualization by classifying all data accumulated in the system. | |||
Revision as of 20:08, 16 July 2022
| Full article title | Laboratory information management system for COVID-19 non-clinical efficacy trial data |
|---|---|
| Journal | Laboratory Animal Research |
| Author(s) | Yoon, Suhyeon; Noh, Hyuna; Jin, Heejin; Lee, Sugyoung; Han, Soyul; Kim, Sung-Hee; Kim, Jiseon; Seo, Jung S.; Kim, Jeong J.; Park, In H.; Oh, Jooyeon; Bae, Joon-Yong; Lee, Gee E.; Woo, Sun-Je; Seo, Sun-Min; Kim, Na-Won; Lee, Youn W.; Jang, Hui J.; Hong, Seung-Min; An, Se-Hee; Lyoo, Kwang-Soo; Yeom, Minjoo; Lee, Hanbyeul; Jung, Bud; Yoon, Sun-Woo; Jang, Hung-Ah; Seok, Sang-Hyuk; Lee, Yu J.; Kim, Seo Y.; Kim, Young B.; Hwang, Ji-Yeon; On, Dain; Lim, Soo-Yeon; Kim, Sol P.; Jang, Ji Y.; Lee, Ho; Kim, Kyoungmi; Lee, Hyo-Jung; Kim, Hong B.; Park, Hun W.; Jeong, Dae G.; Song, Daesub; Choi, Kang-Seuk; Lee, Ho-Young; Choi, Yang-Kyu; Choi, Jung-ah; Song, Manki; Park, Man-Seong; Seo, Jun-Yeong; Nam, Ki T.; Shin, Jeon-Soo; Won, Sungho; Yun, Jun-Won; Seong, Je K. |
| Author affiliation(s) | Seoul National University, RexSoft Corp., Yonsei University College of Medicine, Korea University College of Medicine, International Vaccine Institute, Konkuk University, Seoul National University Bundang Hospital, Chonbuk National University, Korea University, Korea Research Institute of Bioscience and Biotechnology, Kangwon National University, National Cancer Center, Dongguk University, Yonsei University College of Medicine, |
| Primary contact | snumouse at snu dot ac dot kr |
| Year published | 2022 |
| Volume and issue | 38 |
| Article # | 17 |
| DOI | 10.1186/s42826-022-00127-2 |
| ISSN | 2233-7660 |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://labanimres.biomedcentral.com/articles/10.1186/s42826-022-00127-2 |
| Download | https://labanimres.biomedcentral.com/track/pdf/10.1186/s42826-022-00127-2.pdf (PDF) |
|
|
This article should be considered a work in progress and incomplete. Consider this article incomplete until this notice is removed. |
Abstract
Background: As the number of large-scale research studies involving multiple organizations producing data has steadily increased, an integrated system for a common interoperable data format is needed. For example, in response to the coronavirus disease 2019 (COVID-19) pandemic, a number of global efforts are underway to develop vaccines and therapeutics. We are therefore observing an explosion in the proliferation of COVID-19 data, and interoperability is highly requested in multiple institutions participating simultaneously in COVID-19 pandemic research.
Results: In this study, a laboratory information management system (LIMS) has been adopted to systemically manage, via web interface, various COVID-19 non-clinical trial data—including mortality, clinical signs, body weight, body temperature, organ weights, viral titer (viral replication and viral RNA), and multi-organ histopathology—from multiple institutions. The main aim of the implemented system is to integrate, standardize, and organize data collected from laboratories in multiple institutes conducting COVID-19 non-clinical efficacy testing. Six animal Biosafety level 3 (BSL-3) institutions proved the feasibility of our system. Substantial benefits were shown by maximizing collaborative high-quality non-clinical research.
Conclusions: This LIMS platform can be used for current and future outbreaks, leading to accelerated medical product development through the systematic management of extensive data from non-clinical animal studies.
Keywords: SARS-CoV-2, COVID-19, non-clinical, laboratory information management system, data
Background
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in the serious respiratory-related coronavirus disease 2019 (COVID-19) pandemic. It was first reported in Wuhan, the capital of Hubei, China, in late December 2019 and has attracted international attention. [1] After four months, it spread to multiple countries, leading to more than one million confirmed cases worldwide. [2] SARS-CoV-2, a single-stranded RNA-enveloped virus [3], enters the host cells by binding its structural spike (S) protein to cell surface receptors, angiotensin-converting enzyme 2 (ACE2.) [4] After entering the host cells, the virus hijacks the cell to undergo viral replication. [5] During this process, an aberrant host immune condition called a cytokine storm can occur in response to SARS-CoV-2 infection, which is characterized by high concentrations of pro-inflammatory cytokines and chemokines, including tumor necrosis factor-α, interleukin-1, and interleukin-6 [6], resulting in severe COVID-19 associated with excessive inflammation. [7]
Along with understanding the mechanism of transmissibility and pathogenesis of SARS-CoV-2, significant efforts are being devoted to developing novel vaccines and therapeutic agents to prevent or treat COVID-19. In particular, various COVID-19-related research has been conducted with the participation of multiple organizations. Among such studies, animal models of monkeys, cats, ferrets, hamsters, and human angiotensin converting enzyme 2 (hACE2) transgenic mice [8], capable of conducting controlled experiments, have made significant contributions to vaccine development. [9,10,11,12,13] (Note: Though the U.S. Food and Drug Administration doesn't consider animal studies to be part of "non-clinical laboratory studies," many research organizations with animal lab capabilities still use "non-clinical" to include and describe these sorts of animal modelling studies.[1]) Because various parameters such as mortality, clinical signs, body weight, organ weights, viral titer (viral replication and viral RNA), and multi-organ histopathology should be comprehensively analyzed for a reliable interpretation of the results, there is an increasing need for an integrated system in which multiple institutions can apply a streamlined analysis and input the results simultaneously when conducting large-scale non-clinical efficacy tests of a COVID-19 vaccine.
When experimental data are collected from multiple institutions, “organization-level effects” may occur owing to non-standardization in the data coding process and coding errors, which can act as a batch effect in a data analysis. In large-scale experimental multicenter research, it is necessary to prevent the loss of reliability in the data analysis caused by batch effects; however, this is a difficult goal to achieve without an integrated system. An effective approach to overcoming the limitations caused by the prior-mentioned batch effects is to use a laboratory information management system (LIMS). [14] A LIMS systematizes the research process by automating data-related tasks that were conducted manually using information technology, and simultaneously realizes various needs, such as the standardization of experimental data, sharing, problem tracking, and the creation of reports based on document templates. The accumulated data stored in a LIMS can be useful in understanding the current clinical knowledge and making decisions, which is a remarkable advantage in research on rapidly changing epidemics such as COVID-19.
Despite the advantages of a LIMS, no systems allowing multiple institutions to participate simultaneously in research related to COVID-19 have yet been proposed among developed commercial off-the-shelf (COTS) LIMSs. Given that, we aimed to clearly understand the standard procedures of non-clinical trials for COVID-19 therapeutics and vaccines and to design and implement a LIMS suitable for these research purposes.
Results
User management and functionalities
Our COVID-19 non-clinical trial LIMS has four different types of users with different authorizations to handle multiple organizations: a chief director, a local administrator and researcher from each organization, and a pathologist (Fig. 1). The role of the chief director includes new project initiation, modification, deactivation, user account management, and data management. The chief director can also control the registration of new organizations and the removal of existing organizations. The role of the local administrators is the same as that of the chief director, except that they are limited to the organization to which they belong. This enables the efficient management of each organization, as well as data security. Researchers can manage data entry and removal and check the project's progress. Pathologists can conduct pathological analyses, pathological data entry, and related functions. Furthermore, multiple user types for multiple roles are allowed. If multiple authorizations are granted, the screen manual is shown based on the user’s choice.
|
The proposed system, which applies a convenient user interface, was created to provide many functions related to data management for multi-center research. The detailed function list is presented in Table 1.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Functions can be classified into four different categories: access management, trial, audit, and comparison. The access management function forms the basis of the system and manages the user group. The trial function controls the entire project process, including initiation, progression, and finalization. The audit function records all activities that occur in the system and implements data entry, cancelation, and restoration. The comparison function provides several plots according to the investigational product (new drug/repositioning, vaccine/therapeutic), sponsor, experimental animals (hamster model/hACE2 mouse model), research objective, and study site. It performs access control and visualization by classifying all data accumulated in the system.
References
- ↑ "Preclinical and Nonclinical Studies—What Is the Difference, and Where in Your Program Should They Fall?". Premier Consulting. 29 July 2020. https://premierconsulting.com/resources/blog/preclinical-and-nonclinical-studies-what-is-the-difference-and-where-in-your-program-should-they-fall/. Retrieved 27 May 2022.
Notes
This presentation is faithful to the original, with only a few minor changes to presentation, grammar, and spelling. In some cases important information was missing from the references, and that information was added. The authors don't define "non-clinical trial" or "non-clinical research" in the original; for this version, a presumed definition and accompanying citation is provided to the reader for additional context.