Difference between revisions of "LII:A Science Student's Guide to Laboratory Informatics"
Shawndouglas (talk | contribs) (Saving and adding more.) |
Shawndouglas (talk | contribs) (Saving and adding more.) |
||
| Line 163: | Line 163: | ||
===Regulatory compliance=== | ===Regulatory compliance=== | ||
The purpose of placing regulations on laboratory operations varies, but the general idea across them all is to ensure that lab operations are safe, well-managed, and that laboratory data and information are reliable. These regulations cover personnel, their certifications/qualifications, equipment, reagents, safety procedures, and the validity of lab processes, among other things. This includes ensuring that instruments have been calibrated and are in good working order, and that all lab processes have been validated. Laboratory process validation in particular is an interesting aspect and one that causes confusion about what it means and who it applies to. | |||
In the late 1970s, when the original FDA guidelines for manufacturing and laboratory practices appeared, there was considerable concern about what “validation” meant and to what it was applied. The best description of the term is "documented proof that something (e.g., a process) works." The term "validation” is only applied to processes; equipment used during the process has to be "qualified for use." In short, if you are going to carry out a procedure, you have to have documented evidence that the procedure does what it is supposed to do and that the tools used in its execution are appropriate for the need they are to fill. If you are going to generate data and information, do it according to a proven process. This was initially applied to manufacturing and production, including testing and QC, but has since been more broadly used. There were questions about its application to research since the FDA didn’t have oversight over research labs, but those concerns have largely, in industrial circles, disappeared. There is one exception to the FDA's formal oversight in research, which has to do with clinical trials that are still part of the research and development (R&D) process. Product development and production involving human, animal, or food safety, for example, are still subject to regulatory review. | |||
This all culminates down to this: if you you produce data and information, do it through a proven (or even standardized) methodology; how else can you trust the results? | |||
Sources for regulations affecting laboratories include: | |||
* '''FDA''': This includes [[21 CFR Part 11]] of the U.S. Code of Federal Regulations, which helps ensure security, integrity, and confidentially of electronic records.<ref name="CFR21_11">{{cite web |url=https://www.ecfr.gov/current/title-21/chapter-I/subchapter-A/part-11 |title=21 CFR Part 11 Electronic Records; Electronic Signatures |work=Code of Federal Regulations |publisher=National Archives |date=11 November 2023 |accessdate=17 November 2023}}</ref> | |||
* '''EPA''': This includes 40 CFR Part 792 on Good Laboratory Practice Standards<ref name="CFR40_792">{{cite web |url=https://www.ecfr.gov/current/title-40/chapter-I/subchapter-R/part-792 |title=40 CFR Part 792 Good Laboratory Practice Standards |work=Code of Federal Regulations |publisher=National Archives |date=15 November 2023 |accessdate=17 November 2023}}</ref> | |||
==References== | ==References== | ||
Revision as of 22:11, 17 November 2023
Title: A Science Student's Guide to Laboratory Informatics
Author for citation: Joe Liscouski, with editorial modifications by Shawn Douglas
License for content: Creative Commons Attribution-ShareAlike 4.0 International
Publication date: November 2023
|
|
This article should be considered a work in progress and incomplete. Consider this article incomplete until this notice is removed. |
Introduction
An undergraduate science education aims to teach people what they need to know to pursue a particular scientific discipline; it emphasizes foundational elements of the discipline. In most cases in current science education, the time allotted to teaching a scientific discipline is often insufficient to address the existing and growing knowledge base and deal with the multidisciplinary aspects of executing laboratory work in both industrial and academic settings; the focus is primarily on educational topics. Yet employers in science-based industries want to hire people "ready to work," leaving a significant gap between the goals of science education and the background needed to be productive in the workplace. One example of this gap is found in the lack of emphasis on laboratory informatics in laboratory-adjacent scientific endeavors.
The purpose of this guide is to provide a student with a look at the informatics landscape in industrial labs. The guide has two goals:
- Provide a framework to help the reader understand what they need to know to be both comfortable and effective in an industrial setting, giving them a starting point for learning about the product classes and technologies; and,
- Give an instructor an outline of a survey course should they want to pursue teaching this type of material.
This guide is not intended to provide a textbook-scale level of discussion. It's an annotated map of the laboratory portion of a technological world, identifying critical points of interest and how they relate to one another, while making recommendations for the reader to learn more. Its intent is that in one document you can appreciate what the technologies are, and if you hear their names, you'll be able to understand the technologies' higher-level positioning and function. The details, which are continually developing, will be referenced elsewhere.
Note that this guide references LIMSforum.com on multiple occasions. LIMSforum is an educational forum for laboratory informatics that will ask you to sign in for access to its contents. There is no charge for accessing or using any of the materials; the log-in is for security purposes. Sign-in to LIMSforum can be done with a variety of existing social media accounts, or you can create a new LIMSforum account.
What's lacking in modern laboratory education?
The science you’ve learned in school provides a basis for understanding laboratory methods, solving problems, conducting projects/research, and developing methods. It has little to do with the orchestration of industrial lab operations. That role was first filled by paper-based procedures and now firmly falls in the realm of electronic management systems. So why is your laboratory experience in your formal education different from that in industrial labs? After all, both include research, testing, chemistry, biotechnology, pharmaceutical development, material development, engineering, etc.
Educational lab work is about understanding principles and techniques, developing skills by executing procedures, and conducting research projects. Industrial work is about producing data, information, materials, and devices, some supporting research, others supporting production/manufacturing operations. Those products are subject to both regulatory review and are subject to internal guidelines. Suppose a regulatory inspector finds fault with a lab's data. In that case, the consequences can range from more detailed inspection and, if warranted, closing the lab or the entire production facility until remedial actions are enacted.
Because of the importance of data integrity and quality, industrial laboratories operate under the requirements, regulations, and standards of a variety of sources, including corporate guidelines, the Food and Drug Administration (FDA), the Environmental Protection Agency (EPA), the International Organization for Standardization (ISO), and others. These regulatory and standard-based efforts aim to ensure that the data and information used to make decisions about product quality is maintained and defensible. It all comes down to product quality and safety, so for example, that the acetaminophen capsule you might take has the proper dosage and is free of contamination.
Outside of regulations and standards, even company policy can drive the desire for timely, high-quality analytical results. Corporate guidelines for lab operations ensure that laboratory data and information is well-managed and supportable. Consider a lawsuit brought by a consumer about product quality. Suppose the company can't demonstrate that the data supporting product quality is on solid ground. In that case, they may be fined with significant damages. Seeking to avoid this, the company puts into place enforceable policy and procedures (P&P) and may even put into place a quality management system (QMS).
As noted, labs are production operations. There is more leeway in research, but service labs (i.e., analytical, physical properties, quality control, contract testing, etc.) are heavily production-oriented, so some refer to the work as "scientific manufacturing" or "scientific production work" because of the heavy reliance on automation. That dependence on automation has led to the adoption of systems such as laboratory information management systems (LIMS), electronic laboratory notebooks (ELN), scientific data management systems (SDMS), instrument data systems (IDS), and robotics to organize and manage the work, and produce results. Some aspects of research, where large volumes of sample processing are essential, have the same issues. Yet realistically, how immersed are today's student scientists in the realities of these systems and their use outside of academia? Are they being taught sufficiently about these and other electronic systems that are increasingly finding their way into the modern industrial laboratory?
Operational models for research and service laboratories
Before we get too deeply into laboratory informatics concepts, we need to describe the setting where informatics tools are used. Otherwise, the tools won’t make sense. Scientific work, particularly laboratory work, is process-driven at several levels. Organizational processes describe how a business works and how the various departments relate to each other. Laboratories have processes operating at different levels; one may describe how the lab functions and carries out its intended purpose, and others detail how experimental procedures are carried out. Some of these processes—accounting, for example—are largely, with a few exceptions, the same across organizations in differing industries. Others depend on the industry and are the basis for requiring industry experience before hiring people at the mid- and upper levels. Still other activities, such as research, depend on the particular mission of a lab within an organization. A given company may have several different research laboratories directed at different areas of work with only the word “research” and some broad generalizations about what is in common. However, their internal methods of operation can vary widely.
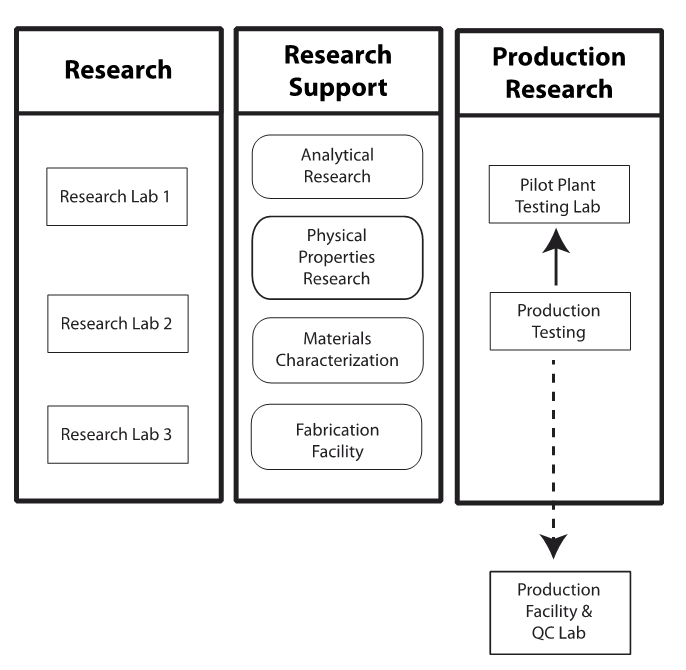
We’ll begin by looking at the working environment. Figure 1 shows the functions we need to consider. That model is based on the author’s experience; however, it fits many applied research groups in different industries whose work is intended to lead to new and improved products. The names of the labs may change to include microbiology, toxicology, electronics, forensics, etc., depending on the industry, but the functional behavior will be similar.
|
The labs I was working in supported R&D in polymers and pharmaceuticals because that's what the overarching company had broad interest in. The research labs (left column of Figure 1) were focused on those projects. The other facilities consisted of:
- Analytical research: This lab had four functions: routine chemical analysis in support of the research labs, new method development to support both research and production quality control (QC), non-routine analytical work to address special projects, and process monitoring that tested the accuracy of the production QC labs (several production facilities were making different products).
- Physical properties research: Similar in function to the analytical lab, this lab measured the physical properties of polymers instead of performing chemical analysis.
- Materials characterization: This group worked with research and special projects looking at the composition of polymers and their properties such as rheology, molecular weight distribution, and other characteristics.
- Fabrication: The fabrication facility processed experimental polymers into blends, films, and other components that could be further tested in the physical properties lab.
Once an experimental material reached a stage where it was ready for scale-up development, it entered the pilot plant, where production processes were designed and tested to see if the material could be made in larger quantities and still retain its desirable properties (i.e., effectively produced to scale). A dedicated testing lab supported the pilot plant to do raw materials, in-process, and post-production testing. If a product met its goals, it was moved to a production facility for larger-scale testing and eventually commercial production.
Intra-lab workflows
Let’s look at each of these support lab groups more closely and examine how their workflows relate.
Analytical research
The workflows in this lab fell into two categories: routine testing (i.e., the service lab model) and research. In the routine testing portion, samples could come from the research labs, production facilities, and the pilot plant testing lab. The research work could come from salespeople (e.g., “We found this in a sample of a competitive product, what is it?”, “Our customer asked us to analyze this," etc.), customer support trying to solve customer issues, and researchers developing test methods to support research. The methods used for analysis could come from various sources depending on the industry, e.g., standards organizations such as ASTM International (formerly American Society for Testing Materials, but their scope expanded over time), peer-reviewed academic journals, vendors, and intra-organizational sources.
Physical properties research
The work here was predominately routine testing (i.e., the service lab model). Although samples could come from a variety of sources, as with analytical research, the test methods were standardized and came from groups like ASTM, and in some cases the customers of the company's products. Standardized procedures were used to compare results to testing by other organizations, including potential customers. Labs like this are today found in a variety of industries, including pharmaceuticals, where the lab might be responsible for tablet uniformity testing, among other things.
Materials characterization
As noted, this lab performed work that fell between the analytical and physical properties labs. While their test protocols were standardized within the labs, the nature of the materials they worked on involved individual considerations on how the analysis should be approached and the results interpreted. At one level, they were a service lab and followed that behavior. On another, the execution of testing required more than "just another sample" thinking.
Fabrication
The fabrication facility processed materials from a variety of sources: evaluation samples from both the production facility and pilot plant, as well as competitive material evaluation from the research labs. They also did parts fabrication for testing in the physical properties lab. Some physical tests required plastic materials formed into special shapes; for example, tensile bars for tensile strength testing (test bars are stretched to see how they deformed and eventually failed). The sample sizes they worked with ranged from a few pounds to thousands of pounds (e.g., film production).
The pilot plant testing lab did evaluations on scaled-up processing materials. They had to be located within the pilot plant for fast turn-around testing, including on-demand work and routine analysis. They also serviced process chromatographs for in-line testing. Their test procedures came from both the chemical and physical labs as they were responsible for a variety of tests on small samples; anything larger was sent to the analytical research labs. The pilot plant testing lab followed a service lab model.
On the service lab model and research in general
The service lab model has been noted several times and is common in most industries. The details of sample types and testing will vary, but the operational behavior will be the same and will work like this:
- Samples are submitted for testing. In many labs, these are done on paper forms listing sample type, testing to be done, whom to bill, and a description of the sample and any unique concerns or issues. In labs with a LIMS, this can be done online by lab personnel or the sample submitter.
- The work is logged in (electronically using a LIMS or manually for paper-based systems), and rush samples are brought to management's attention. Note that in the pilot plant test lab, everything is in a rush as the next steps in the plant’s work may depend upon the results.
- Analysts generate worklists (whether electronic or paper, an ordered representation of sample or specimen locations and what analyses must be performed upon them by a specific instrument and/or analyst using specified procedures) and perform the required analysis, and results are recorded in the LIMS or laboratory notebooks.
- The work is reviewed and approved for release and, in paper systems, recorded on the submission forms.
- Reports are sent to whoever submitted the work electronically, or via the method the submitter requested.
Work from non-routine samples may be logged in under “special projects,” though it may create the need for additional testing.
There is no similar model for research work besides project descriptions, initial project outlines, etc. The nature of the work will change as the project progresses and more is learned. Recording results, observations, plans, etc., requires a flexible medium capable of maintaining notes, printouts, charts, and other forms of information. As a result, ELNs are modular systems consisting of a central application with the ability to link to a variety of functional modules such as graphics, statistics, molecular drawing, reaction databases, user-define database structures to hold experimental data, and more. For additional details, see The Application of Informatics to Scientific Work: Laboratory Informatics for Newbies.
The role of laboratory informatics
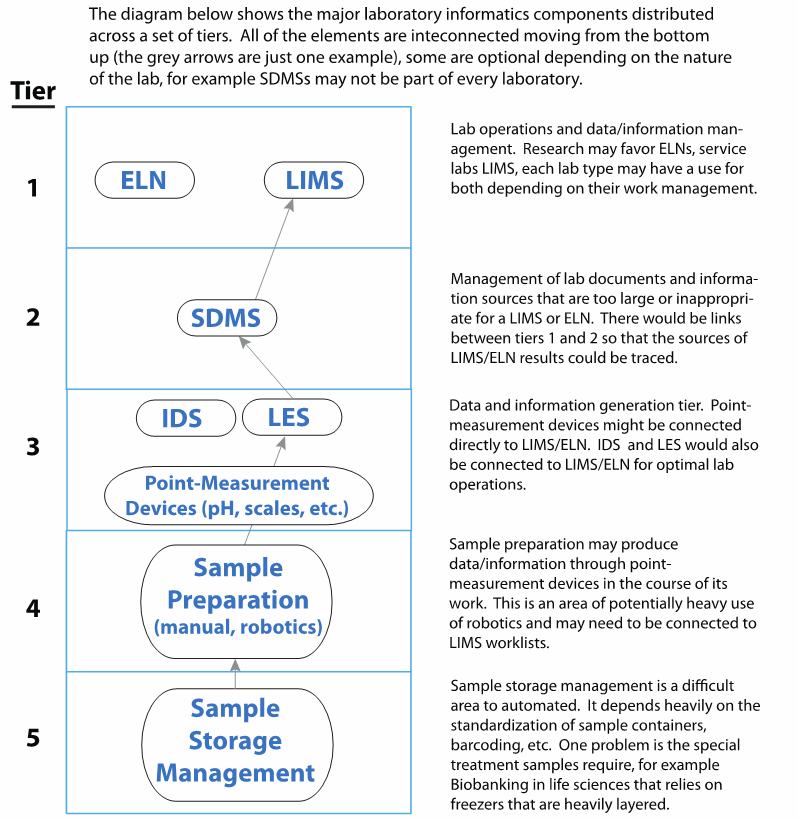
Lab informatics has several tiers of systems (Figure 2) that can be applied to lab work to make it more effective and efficient.
|
The top tier consists of ELNs and LIMS, while supporting those systems are SDMS, as well as laboratory execution systems (LES) and IDS (which are are a combination of instruments and computer systems). Typical examples are chromatography data systems (CDS) connected to one or more chromatographs, a mass spectrometer connected to a dedicated computer, and almost any major instrument in an instrument-computer combination. CDSs are, at this point, unique in their ability to support multiple instruments. Sharing the same tier as LES and IDS are devices like pH meters, balances, and other devices with no databases associated with them; these instruments must be programmed to be used with upper-tier systems. Their data output can be manually entered into a LIMS, ELN, or LES, but in regulated labs, the input has to be verified by a second individual. Below that are mechanisms for sample preparation, management, and storage. Our initial concern will be with the top-tier systems.
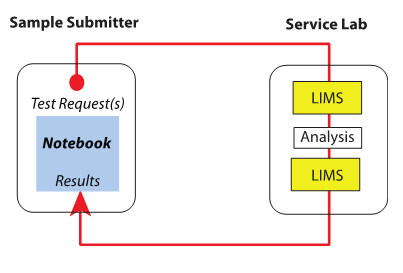
Next we look at the interactions involved in such workflows. The primary interaction between a service lab and someone requesting their services is shown in Figure 3.
|
Samples are submitted by the research group or other groups, and the request proceeds through the system as described above. The split between the LIMS in Figure 2 illustrates the separation between logging samples in, the analysis process, and using the LIMS as an administrative tool for completing the work request and returning results to the submitter. Also note that Figure 2 shows the classical assignment of informatics products to labs: LIMS in service labs and notebooks (usually ELNs) to research labs. However, that assignment is an oversimplification; the systems have broader usage in both types of laboratory workflows. In the research labs, work may generate a large amount of testing that has to be done quickly so that the next steps in the experiments can be determined. The demand may be great enough to swamp the service labs, and they wouldn't be able to provide the turn-around time needed for research. In these cases, a LIMS would be added to the research lab's range of informatics tools so that high-demand testing would be done within those labs. Other, less demanding testing would be submitted to the service labs. The research lab LIMS could be an entirely independent installation or work with the same core system as the service labs. The choice would depend on the locations of the labs, the need for instrument connections, how cooperative they are, and corporate politics.
The analytical research and materials characterization labs in Figure 1 could justify an ELN based on research associated with method development work. In addition to providing a means of detailing the research needed to create a new procedure, the ELN would need access to a variety of databases and tools, including chemical reaction modeling, molecular structure representation, published methods retrieval, etc., as well as any corporate research databases that could exist in a large organization.
The fabrication facility could use an ELN to record the progress of any non-routine work that was being done. Equipment operating conditions, problems encountered and solved, and the results of their processing would be noted. This could be coordinated with the pilot plant or product development group’s work.
Laboratory operations and their associated informatics tools
Laboratory operations and work can be divided into two levels:
- Data and information generation: This is where lab procedures are executed; the informatics tools consist of LES, IDS, and support for automation and devices such as pH meters, scales, etc.
- Data, information, and operations management: This involves the management of the data and information ultimately generated, as well as the operational processes that led up to the creation that data and information. The informatics tools at this level consist of LIMS, ELN, and SDMS.
The following largely addresses the latter, recognizing that the execution of analytical procedures—both electronically and manually—results in the creation of data and information that must be properly managed. Data, information, and operations management (i.e., laboratory management) involves keeping track of everything that goes on in the lab, including:
- Lab personnel records: The qualifications, personnel files (along with human resources), vacation schedules, education/training, etc. of the lab's personnel.
- Equipment lists and maintenance: Records related to scheduled maintenance, repairs, calibration, qualification for use, and software upgrades, if appropriate.
- General process-related documentation: All lab documents, reports, guidelines, sample records, problems and non-conformities, method descriptions, contacts with vendors, etc.
- Sample-specific records: What samples need work, what is the scope of the work, results, associated reports, etc.
- Inventory: Records related to what materials and equipment (including personal protective equipment) are on hand, but also where those material and equipment are, their age (some materials such as prepared reagents have a limited useful lifetime, in other cases materials may have a limited shelf-life), and any special handling instructions such as storage and disposal.
- Chemical and organism safety: Any special conditions needed for chemicals and organisms, their maintenance, condition, related records (e.g., material safety data sheets), etc.
- Data governance efforts
- Data integrity efforts
- Regulatory compliance efforts: Documentation regarding laboratory effort to meet regulatory requirements or avoid regulatory issues, including preparation for regulatory audits.[1]
Many of those laboratory operational aspects will be familiar to you, others less so. The last three bullets may be the least familiar to you. The primary point of lab operations is to produce data and information, which will then be used to support production and research operations. That data and information must be reliable and supportable. People have to trust that the analysis was performed correctly and that it can be relied upon to make decisions about product quality, to determine whether production operations are under control, or to take the next step in a research program. That’s where regulatory compliance data governance, data integrity, and regulatory compliance come in.
Data governance
Data governance refers to the overall management of the availability, usability, integrity, and security of the data employed in an enterprise. It encompasses a set of processes, roles, policies, standards, and metrics that ensure the effective and efficient use of information in enabling an organization to achieve its goals. Its primary purpose is to ensure that data serves the organization's needs and that there's a framework in place to handle data-related issues.
Data governance-related efforts have become more important over time, especially in how data and metadata are stored and made available. We see this importance come up with recent frameworks such as the FAIR principles, that ask that data and metadata be findable, accessible, interoperable, and reusable.[2]
Data integrity
Data integrity refers to the accuracy and consistency of data over its lifecycle. It ensures that data remains unaltered and uncorrupted unless there's a specific need for a change. It’s focused more on the validity and reliability of data rather than the broader management processes. Its primary purpose is to ensure that data is recorded exactly as intended and, upon retrieval, remains the same as when it was stored.[3]
While data governance is a broader concept that deals with the overall management and strategy of data handling in an organization, data integrity is specifically about ensuring the accuracy and reliability of the data. Both are essential for making informed decisions based on the data and ensuring that the data can be trusted.
The topic of data integrity has also increasingly become important.[3] A framework called ALCOA+ (fully, ALCOA-CCEA) has been developed by the FDA to help define and guide work on data integrity. ALCO+ asks that paper and electronic data and information be attributable, legible, contemporaneous, original, accurate, complete, consistent, enduring, and available, which all further drive data integrity initiatives in the lab and beyond.[4] For more on this topic, see Schmitt's Assuring Data Integrity for Life Sciences.[3]
Regulatory compliance
The purpose of placing regulations on laboratory operations varies, but the general idea across them all is to ensure that lab operations are safe, well-managed, and that laboratory data and information are reliable. These regulations cover personnel, their certifications/qualifications, equipment, reagents, safety procedures, and the validity of lab processes, among other things. This includes ensuring that instruments have been calibrated and are in good working order, and that all lab processes have been validated. Laboratory process validation in particular is an interesting aspect and one that causes confusion about what it means and who it applies to.
In the late 1970s, when the original FDA guidelines for manufacturing and laboratory practices appeared, there was considerable concern about what “validation” meant and to what it was applied. The best description of the term is "documented proof that something (e.g., a process) works." The term "validation” is only applied to processes; equipment used during the process has to be "qualified for use." In short, if you are going to carry out a procedure, you have to have documented evidence that the procedure does what it is supposed to do and that the tools used in its execution are appropriate for the need they are to fill. If you are going to generate data and information, do it according to a proven process. This was initially applied to manufacturing and production, including testing and QC, but has since been more broadly used. There were questions about its application to research since the FDA didn’t have oversight over research labs, but those concerns have largely, in industrial circles, disappeared. There is one exception to the FDA's formal oversight in research, which has to do with clinical trials that are still part of the research and development (R&D) process. Product development and production involving human, animal, or food safety, for example, are still subject to regulatory review.
This all culminates down to this: if you you produce data and information, do it through a proven (or even standardized) methodology; how else can you trust the results?
Sources for regulations affecting laboratories include:
- FDA: This includes 21 CFR Part 11 of the U.S. Code of Federal Regulations, which helps ensure security, integrity, and confidentially of electronic records.[5]
- EPA: This includes 40 CFR Part 792 on Good Laboratory Practice Standards[6]
References
- ↑ Liscouski, J. (June 2023). "Harnessing Informatics for Effective Lab Inspections and Audits" (PDF). LabLynx, Inc. https://www.lablynx.com/wp-content/uploads/2023/06/Article-Harnessing-Informatics-for-Effective-Lab-Inspections-and-Audits.pdf. Retrieved 17 November 2023.
- ↑ Wilkinson, Mark D.; Dumontier, Michel; Aalbersberg, IJsbrand Jan; Appleton, Gabrielle; Axton, Myles; Baak, Arie; Blomberg, Niklas; Boiten, Jan-Willem et al. (15 March 2016). "The FAIR Guiding Principles for scientific data management and stewardship" (in en). Scientific Data 3 (1): 160018. doi:10.1038/sdata.2016.18. ISSN 2052-4463. PMC PMC4792175. PMID 26978244. https://www.nature.com/articles/sdata201618.
- ↑ 3.0 3.1 3.2 Schmitt, S. (2016). Assuring Data Integrity for Life Sciences. DHI Publishing, LLC. ISBN 1-933722-97-5. https://www.pda.org/bookstore/product-detail/3149-assuring-data-integrity-for-life-sciences.
- ↑ "Data Integrity for the FDA Regulated Industry" (PDF). Quality Systems Compliance, LLC. 12 January 2019. https://qscompliance.com/wp-content/uploads/2019/01/ALCOA-Principles.pdf. Retrieved 17 November 2023.
- ↑ "21 CFR Part 11 Electronic Records; Electronic Signatures". Code of Federal Regulations. National Archives. 11 November 2023. https://www.ecfr.gov/current/title-21/chapter-I/subchapter-A/part-11. Retrieved 17 November 2023.
- ↑ "40 CFR Part 792 Good Laboratory Practice Standards". Code of Federal Regulations. National Archives. 15 November 2023. https://www.ecfr.gov/current/title-40/chapter-I/subchapter-R/part-792. Retrieved 17 November 2023.