LII:The Practical Guide to the U.S. Physician Office Laboratory
The Practical Guide to the U.S. Physician Office Laboratory
Written by: Rebecca A. Fein, M.S.A.H.I., M.B.A.
Edited by: Shawn Douglas
Introduction
This guide intends to give the reader practical information on the physician office laboratory (POL), assisting the reader with the decision-making processes related to becoming affiliated with a POL.
This guide provides a discussion on the history and trends related to the POL market, as well as testing considerations, staffing requirements, regulatory issues, related technology, and economic considerations.
No recommendations are made, though appropriate best practices are mentioned.
What is a physician office laboratory?
The definition of a physician office laboratory varies from state to state. Some states define the POL by the actual number of physicians in the practice, and others do not. When setting up a POL, the proprietor should consult their individual state regulatory body to ensure full compliance.
For the purpose of this paper, the definition provided by the State of New York is used, as New York regulations are strict, and using a stringent guideline for the rest of this guide is preferred:
In order to qualify as a physician office laboratory (POL), individual health care providers must operate the practice or be part of a legally constituted, independently owned, and managed partnership or group practice. Laboratories that are owned, managed and/or operated by managed care organizations, hospitals or consulting firms do not qualify for the POL exception and should apply for a clinical laboratory permit through the Clinical Laboratory Evaluation Program.[1]
Based on the New York definition, a POL is thus a laboratory that provides the physician in-office laboratory testing services, thereby allowing the physician to have quicker access to laboratory results. Depending on the rules and regulations in any given state, the lab can be owned and operated either by a single physician practice or by more than one physician practice. In many states, additional regulations prohibit the acceptance of specimens from outside the clinician's practice.
Types of POLs and their workflow
POLs come in many different types. Nearly any practice can operate a POL. However, the most common types include gastroenterology, family practice, internal medicine, obstetrics and gynecology, and hematology and oncology practices. This is likely due to the need for these specialties to get quick results for treatment plan decisions.
According to United Healthcare's Oxford's In-Office Laboratory Testing and Procedures List, the specialists that use an in-office laboratory include[2]:
- Primary care physicians and specialists: In some parts of the world, this type of doctor is referred to as a general practitioner. This doctor is the first point of contact for a patient and coordinates referrals to other physicians as necessary (dependent upon the insurance plan a patient has). Often these doctors are family medicine or internal medicine specialists by training. In some cases, these doctors are OB/GYNs, nephrologists, allergists, pediatricians, or emergency medicine specialists.
- Dermatologists / dermatopathologists: A dermatologist is a doctor who specializes in skin conditions. Dermatologists can also board certify as dermatopathologists, which are trained in both dermatology and pathology. A dematopathologist examines tissue samples taken as part of a biopsy, for example.
- Rheumatologists: Rheumatologists deal with issues related to joints, soft tissue, autoimmune diseases, vasculitis, and hereditary connective tissue disorders.
- Urologists: Urologists examine issues related to the male and female urinary tract, as well as the male reproductive tract.
- Pediatricians: Pediatricians are trained to treat medical conditions found in infants, children, and adolescents. Generally, they do not see patients over the age of 18.
- Pulmonologists: Pulmonologists specialize in conditions related to the respiratory tract.
- Hematologists / oncologists / pediatric hematologists: Hematologists specialize in blood-related conditions, oncologists in cancers, and pediatric hematologists in childhood blood disorders.
- Obstetricians / gynecologists: Obstetricians specialize in conditions related to pregnancy. Often they are also gynecologists, specializing in women’s reproductive health conditions.
- Reproductive endocrinologists / infertility: This is a sub-branch of the above specialty, addressing both male and female infertility issues.
Other specialists such as surgeons are not as likely to have a physician office lab. Surgeries often take place in a hospital, and therefore tests would be processed through the hospital lab.
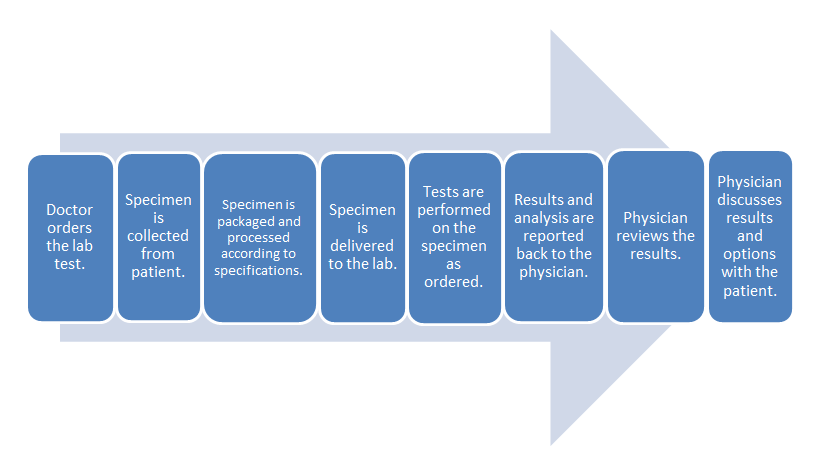
- Common clinical laboratory workflow:
- POL workflow:
The difference in these two workflows mostly comes down to the time spent in transporting the specimen to an outside lab and waiting for the processing. The in-office lab saves time in those parts of the process.
History and market trends of the POL
In ancient times, patient diagnosis predicated on what the physician could observe during an exam of the patient, and in some cases the samples from the patient.[3]
Beginning in the Middle Ages and ending during the eighteenth century, bed side medicine was the predominant form of practice. Patients were diagnosed at their bed side, similar to what modern clinicians call point-of-care testing (POCT).[3]
During the eighteenth and nineteenth centuries, with the rise of hospital medicine, laboratory medicine started to play a bigger role in the diagnosis of patients.[3]
Although, instruments such as the stethoscope and thermometer came into use at the end of the nineteenth century, the clinical laboratory did not become a standard part of medical practice until the twentieth century.[4]
Early diagnostic testing
Diagnosis of patients via techniques, such as the examination of bodily fluids to predict disease, can be traced back to Hippocrates in ancient Greece. Hippocrates instituted diagnostic criterion that included listening to the patient’s lungs, examining the urine of the patient, and observing the patient’s skin color.[3]
Blood in urine was linked to kidney failure in 50 AD, followed by another early clinician, Galen, identifying diabetes as “diarrhea of urine” and noting a normal relationship between fluid intake and urinary output in 180 AD.[3]
In 900 AD, Isaac Judaeus established a protocol for using urine in patient diagnoses [3]. By 1300 AD, examination of the urine under a microscope (uroscopy) had become so popular it was nearly universal in Europe.[3]
The seventeenth century saw many innovations in diagnostic techniques as a result of the advances in literature related to the structure of the body and the formation of scientific societies.[3] Some of the innovations that came from the seventeenth century include first attempts to use pulse and temperature as indications of illness in a patient; intravenous drug injections; and identification of the sweet taste of urine in patients with diabetes.[3]
In the eighteenth century, Dr. William Hewson began to identify ways to measure coagulants in blood tests, an event that set the stage for modern diagnostic laboratory practice. During this time period, the ability to use temperature and blood pressure as diagnostic indicators was refined, allowing James Currie to treat his typhoid fever patients by putting them in a cold bath.[3]
Other advances out of the eighteenth century include Sir John Floyer’s pulse measuring technique, Tichy’s urine analysis technique, Dobson’s ability to prove the sweetness of both blood and urine in patients with diabetes was caused by sugar, and Home's development of a yeast test for sugar in urine.[3]
The nineteenth century is sometimes referred to as the era of public health; during this time independent laboratories started to develop.[3] In the United States, laboratory medicine was viewed with skepticism, as a destructive force related to medical knowledge. As a result of this, many American physicians went to Europe to receive training on laboratory techniques.[3]
As older physicians retired from practice and faculty positions, opposition to laboratory practice faded, allowing for bacteriological discoveries like pasteurization.[3] The nineteenth century saw aseptic methodologies produce fewer deaths after surgery, resulting in a greater emphasis on hygienic practices. This is also the period of time when x-ray and microscopy become more important to the practice of medicine.[3]
Around 1850, the laboratory became popularized, and the first hospital laboratories begin to appear. Prior to this time period, most laboratory tests were performed at the bed side or were performed by the physician in the office laboratory.[3]
Diagnostic testing in the twentieth century
Although the nineteenth century was a time of advancement for clinical laboratory practice, the blossoming of the clinical laboratory did not occur until the twentieth century.[4] In the early twentieth century, laboratories began to stratify into the different types of sub-specialties seen in practice today: public health, forensic, and clinical.[4]
In 1928, when Alexander Fleming accidentally discovered penicillin, he ushered in the age of antibiotics, which allowed for new treatment options for infections, especially when combined with Domagk’s discovery that sulphonomides had antibacterial attributes and did not harm humans.[4]
In the twentieth century, laboratory medicine personnel needed to be certified and licensed as the movement to ensure quality in medicine came to the laboratory. Organizations were founded to accommodate this, including the American Society for Clinical Pathology (ASCP), founded in 1922 to offer certifications to pathologists.[4]
By the end of the 1950s, the clinical laboratory had earned the respect of other medical professionals and the public, ensuring professional legitimacy.[4] This was accomplished by the discoveries made in the clinical laboratory, leading to new treatment options for patients, who would have endured difficult illnesses, or died, without such interventions.[4]
The initial creation of Medicare in 1965 was seen as an opportunity for free money by the healthcare industry as a whole. As costs increased, loopholes were found to get more reimbursements; however, these loopholes would be closed, and the resourceful provider would find other ways to continue the practice of charging more to get a higher reimbursement.[5]
The government soon discovered the potential for fraud and abuse inherent in Medicare. The need for regulations in order to prevent such abuses became apparent as early as 1967.[5] The Clinical Laboratory Improvement Act came into effect that year as a tool to regulate laboratories practicing across state lines, and it was amended in 1988 to include nearly all laboratories practicing in the United States.[5]
In 1989, an estimated 98,400 POLs were operating in the United States. Estimates from the time vary from 20,000 to 200,000 due to the lack of a standard definition for a POL and the need for physicians to self-report the status of their lab.[6] Some of these issues continue to persist today, as states often have different definitions for a POL.
In 1989, limited regulatory controls existed for POLs, resulting in wide variations in the complexity of testing among POLs.[6] Kusserow, writing for the HHS Office of Analysis and Inspections, noted the following during this time period:
Physicians operating office laboratories conduct approximately 25 percent of all laboratory testing in the country. Sixteen States have laws pertaining to them. About $20 billion is spent nationally on laboratory services annually, of which POLs receive $5 billion. Each year Medicare pays POLs over $400 million.[6]
Kusserow also found the average 1985 Medicare Part B payment to POLs was $7 per test, compared to $10 per test for an independent lab, or $19 per test to a lab classified as "other".[6] One can see the trend: the POL became a cheaper option when compared to other types of labs.
Since 1995, with a better understanding and acceptance of regulations on the laboratory and the list of waived tests growing from 8 to 40[7], the number of POLs in the United States increased to a total of 120,399 or 49% of all the laboratories in the United States as of December 2013.[8] Additionally, 60% of the POLs in the United States today are running Clinical Laboratory Improvement Amendments (CLIA) waived tests, and 24% hold provider performed microscopy (PPM) certificates.[9]
According to Bachman, POL growth is expected to increase in the future due to an aging population of baby boomers with money to finance laboratory testing and an increased interest and awareness in healthcare topics. Other factors to watch out for in the future are a softening stance on testing by payors (an example of this is the addition of an initial physical exam given to new Medicare beneficiaries for preventative care) and additions to the list of CLIA waived tests.[7]
Why do POLs exist?
Historically, POLs are a subset of point-of-care testing (POCT), laboratory testing that is done where the patient is located. These laboratories came about initially because as clinical medicine became more complicated to practice and techniques became more sophisticated, physicians still needed to perform tests to diagnose patients.
POLs also exist because the industry was looking for a cheaper way to test. Running a POL was found to be an effective way to provide clinician information for treatment plans, while at the same time saving insurers money.
In some cases, POLs opened to provide additional revenue streams for a physician’s practice. The reasons for the POL's existence are varied, but the central reason is to provide quality diagnoses, treatment, and care to patients in the healthcare system.
Advantages and disadvantages of running a POL
In the early days of the POL, lack of regulation proved to be a disadvantage; however, over time, regulations have caught up for the better.
Advantages include
- quicker access to test results for the clinician, leading to more treatment options for the patient;
- greater efficiency of the clinical workflow;
- cheaper testing, though subject to individual test and pricing information; and
- patient comfort and happiness, including time saved by having to go only to one location.
Disadvantages include:
- the physician office being the only point-of-access, with some physicians not willing to release patient information to an outside party (such as a hospital or competing clinician). This disadvantage may be eliminated due to regulatory changes in April of 2014, now allowing patients direct access to their laboratory results;
- patients not feeling comfortable about the physician's office being the central repository of information, and physicians may not see the value in having a lab in their practice; and
- the cost of meeting compliance requirements for local, state, and federal regulations, especially in states with stricter requirements.
These lists are of course limited; one could weigh advantages and disadvantages endlessly if the appropriate time was spent to fully evaluate the endeavor. Some of this process would be related to the individual practice in question.
How the POL integrates with the entire practice
POLs can integrate with an entire practice in a variety of ways. First, POLs can store laboratory data in a form more readily exchanged between the laboratory and the patient's broader electronic health record (EHR). A slight disconnect often exists between reference labs and physician offices. By placing a lab in the physician office, tighter integration of patient and testing data is achieved, a benefit for both the patient and the practitioner.
The tighter integration may save patients follow-up visits for diagnoses that are able to be done in the office laboratory. For example, in diagnosing a urinary tract infection, the physician office can significantly reduce time spent sending the sample off for testing by doing the testing independently.
Additionally, the POL can allow the financial departments of a practice to track costs and revenue by using laboratory data. For example, during flu season the physician can budget more money for gloves if their data indicates they're seeing more patients during this time.
Laboratory data can also assist with trends related to a population. If a POL notices an unexpected trend in disease among the patient population, the lab data can help the entire organization decide how best to address the related issues through community education or some other outreach program.
POL or reference lab?
On average, most POL testing is simple and basic, falling under what is known as Clinical Laboratory Improvement Amendments (CLIA) waived tests. These tests will be discussed further in the next section, but for now know they are near the patient and simple to perform. As previously discussed, bringing simple laboratory testing to the physician office provides benefits to both the patient and the physician, making the POL more attractive.
For some physicians a POL is best because of their location; they may operate in a rural environment and would not have access to laboratory services if they did not do it themselves. For others, the expense of creating such a lab has swayed them towards using a reference lab, which can perform complex tests and, in many cases, has a staff available 24/7. And while placing a POL in the physician office may integrate patient and lab data better, software-based offerings like Health Gorilla that provide real-time results may be sufficient for physicians that prefer to use a reference lab.
In the end, the decision to set up a POL or use a reference lab is based on a review of the advantages and disadvantages, finding a balance of what is best for both the patients' interest and the practice's long-term stability.
Testing and associated reporting
CLIA lays out seven criteria for determining the complexity of a test, including the origin of the test.[10] For example, if a new test is developed or an existing test is modified, and then it's used at that laboratory, the test is automatically rated a high-complexity test.[10] The complexity of the test determines the requirements the laboratory needs to comply with in order to maintain regulatory compliance. The more complex the test is, the stricter the requirements are.[10]
Test complexity has three levels: high, moderate, and waived. Waived tests are simple to perform and have a relatively low risk of an incorrect test result.[10] Moderately complex tests include tests like provider performed microscopy (PPM), which requires the use of a microscope during the office visit.[10] Providers that want to perform PPM tests must be qualified to do so under CLIA regulations.[10]
High-complexity tests require the most regulation. These tests are the most complicated and run the highest risk of an inaccurate result, as determined during the FDA pre-market approval process.[10] Tests may come from the manufacturer with their complexity level on them, or one can search the FDA database to determine the complexity of the test.[10] It is important to understand the complexity level of the testing provided in order to ensure full compliance with CLIA.
Commonly performed tests, according to the United Healthcare guide, include[2]:
- urine analysis
- urine pregnancy
- blood occult
- glucose blood
- pathology consultation during surgery
- crystal identification by microscope
- sperm identification and analyses
- bilirubin total
- blood gasses
- complete blood count
- bone marrow smear
- blood bank services
- transfusion medicine
Reimbursement levels for tests are dependent upon the reimbursement guide put out by the individual insurance company. Billing personnel in POL-related offices are ultimately responsible for finding out what the reimbursement is and which tests are permitted for the POL's level of certification.
As noted, the predominant form of testing in the POL is waived complexity testing. See Table 1 in the appendix for the complete list of waived tests as of May 2014.
Reporting
Just as POLs manage a set of commonly performed tests, a set of corresponding reports provides the results of those tests. The results will pass through a set of validation and quality control checks (discussed in section 8.2) before being fashioned into a final report for the ordering physician. For example, if a complete blood count is ordered by the physician, a corresponding patient report is produced by the laboratory, often through a laboratory information system (LIS). The patient report contains patient, physician, and sample demographics, as well as the results and whether they are above, below, or within recommended limits. Other types of reports may be generated in the laboratory, including daily summary, test total, and various quality control reports.
Staffing and certification requirements for the POL
Regulatory requirements regarding laboratory staff vary depending upon the level of testing performed and the state the POL is operating in, and due diligence is required to ensure the POL is meeting those requirements. The previous section noted most POLs perform waived tests, and as such, this section focuses on the requirements for staffing a CLIA waiver-certified laboratory. Typically these labs operate only during office hours, and therefore they do not have 24/7 staffing.
The highest level of laboratory management is typically the person holding the title of laboratory director. Laboratory directors are responsible for the administration and operations of the laboratory, including the hiring of all personnel and ensuring testing procedures are done in the correct manner.[11] For the waiver-certified laboratory, anyone may be a laboratory director; however, the Joint Commission recommends laboratory directors meet the same minimum requirements of those testing in moderately complex laboratories.[12] Some states require the laboratory director of a moderately complex laboratory to be state-licensed and also to be a medical doctor or hold a degree in a laboratory science, such as chemistry.[13]
In general, the laboratory staff of a POL may consist of the laboratory director and a mix of laboratory technicians, laboratory technologists, laboratory clinical consultants, and, in some cases, a laboratory manager. The presence of some of these roles may vary, dependent upon if the laboratory is certified for waived, moderately complex, or highly complex testing. Additional educational qualifications and certifications for the laboratory technician, histotechnician, laboratory director, and other staff members may exist based on the lab's complexity level. Directors should check state requirements for the type of lab in question to ensure full compliance.
Regulatory requirements and considerations
When setting up a POL, the proprietor is faced with individual state, local, and federal regulations to ensure full compliance. (This guide is no substitute.) Three key regulations were chosen for discussion in this guide: the Clinical Laboratory Improvement Amendments (CLIA), the Health Insurance Portability and Accountability Act (HIPAA), and the Patient Protection and Affordable Care Act (PPACA). These regulations were chosen because they are the most common — and most important — to affect the POL and the physician office.
CLIA
The most impactful regulation for the physician office laboratory at the time of this writing was CLIA. The U.S. federal statute was implemented in 1988 to remove obsolete laboratory requirements and include new requirements to improve the quality of a modern clinical laboratory.
As previously noted, most POLs are CLIA waiver labs, and therefore much like the previous section, the discussion here is mostly centered on those requirements. Waived tests have a low risk of an incorrect result; this includes the tests the Food and Drug Administration (FDA) has approved for consumers to use in their homes.[10] Tests performed under this provision are done at laboratories that have registered as required by CLIA and obtained a certificate of waiver. These labs are not inspected on a routine basis like labs certified to perform moderate- and high-complexity testing. Laboratories that wish to change their status from waived to one of the other statuses must comply with the CLIA requirements for registration, inspection, and proficiency testing as outlined in the law.[10] Waived laboratory staff, as previously mentioned, does not require proficiency testing, and anyone can be qualified to be the laboratory director.[10]
HIPAA
POLs are required to comply with HIPAA and must provide safeguards for the security and privacy of the data collected and maintained in the laboratory. HIPAA passed in 1996 in an attempt to provide better guidance regarding the privacy and security of data, portability of health insurance, and better accountability for violations related to these topics. Laboratories are required to implement measures that prevent the unauthorized disclosure and access to data in the laboratory.
Prior to 2014, most laboratories were exempt from the HIPAA requirement to provide patients with lab results or other protected health information.[14] However, in February 2014, the Department of Health and Human Services wanted to provide patients the opportunity to become better members of their own care team by giving them more information about their health.[14] This resulted in the amendment of CLIA 1988 to require a laboratory to give a patient, or their designated representative, lab results within 30 days of said individual sending a written request.[14] Laboratories are still required to ensure those accessing this data have authorization to do so, as the original requirements to keep data secure and private remain the same.
The annual cost of compliance with this new rule is estimated to be $59 million over the first five years when examining the potential cost to the laboratory industry in general.[15] Laboratories like Quest Diagnostics support the rule because it will allow them to give patients their lab results without prior approval from the patient’s provider.[15]
As of this writing, it remains unclear as to how this impacts the POL. Since the POL is located at the physician office, access to results is most likely determined by the provider’s regular procedures for acquiring personal health information (PHI). The POL could provide forms to patients for release of PHI, just as any other lab can, but it is unclear as to how this rule change will impact the POL in the long term.
PPACA
As of this writing, the most difficult regulation to assess is the Patient Protection and Affordable Care Act, also known as the ACA. This does not negate the obligations of the laboratory under CLIA and HIPAA. According to the Clinical Laboratory Coalition, laboratory testing informs about 70% of a clinician’s medical decision-making process. However, the laboratory comprises less than two percent of Medicare spending.[16]
The Patient Protection and Affordable Care Act (PPACA) included a direct cut to the Medicare Clinical Laboratory Fee Schedule of 1.75 percent each year for 5 years. This 9 percent cut is the largest cut among all Part B providers and started in 2011. In PPACA, clinical laboratories also received another cut in the form of a productivity adjustment, resulting in an additional 11 percent cut over 10 years.
The laboratory-specific cut and the productivity adjustment will already result in a cumulative 20 percent cut over 10 years. Laboratories are also facing up to a 2 percent cut to the fee schedule as a result of sequestration, which begins in January 2013.[16]
The laboratory space in general may face challenges from the accountable care organization (ACO) model under the PPACA, due to a decrease in laboratory testing volume.[17] Under the ACO model, unnecessary or redundant testing would be discouraged.[17] This could be a good thing for the POL market, as waived testing would be done in-house, close to the patient. It could also be a problem for the POL market, as physicians may need to recalculate if operating a POL makes economic sense for their practice.
Economic considerations
Economics are very important to any aspect of the medical practice. This is also true for the POL. Four economic considerations should be made regarding the POL: profitability and stability, insurance reimbursements, billing, and return on investment (ROI). For example, at the end of the previous section, the economics surrounding the PPACA were discussed. Other considerations would include things like assessing financial penalties for non-compliance.
Profitability and sustainability of the POL
Maintaining a profitable and sustainable lab is important. This is why the ROI calculation is provided in section 7.4 for examination. Performing this calculation can help determine how long it will take the lab to become profitable and if it will be sustainable over time. Like most parts of a business, the laboratory becomes profitable by collecting more revenue than it is putting out in expenses.
Insurance reimbursements
Insurance reimbursements vary by insurance company and plan. Checking with the insurance companies and plans accepted by the POL is advised; these numbers often change, as required by regulations or insurance company needs. It is important to keep current on this subject. Failing to do so can result in less reimbursement than one is expecting. If the reimbursement for a test is cut from $25 to $5, and one fails to keep current with reimbursements from the insurance providers, the shock could ripple through the laboratory or provider practice.
The April 2014 passage of the “Doc Fix,” a one-year protection of the Sustainable Growth Rate (SGR) Medicare physician payment formula, saved providers from this type of shock[18], as without this legislation providers were set to experience a 30% reduction in their reimbursement rates.[16] Laboratory personnel, especially billing personnel, would be wise to keep up with trends in this area.
Billing
Billing requires medical codes such as the International Classification of Disease, Ninth Version, Clinical Modification (ICD-9-CM) codes, soon to be ICD-10-CM codes, as well as procedure codes from either the Current Procedural Terminology (CPT) codes or the Healthcare Common Procedure Coding System (HCPCS). Logical Observation Identifiers Names and Codes (LOINC) may also be used, but mapping to another code set such as CPT is more common. Ordering physician, patient name, medical record number (MRN), and other demographic information may also appear on billing. Laboratory-specific information such as CLIA certification number and modifications indicating whether the test is CLIA-waived are also present.
Laboratory billing for Medicare went through an important simplification process in 2003, to allow for a more standardized process and to eliminate confusion office staff was experiencing in their attempts to comply with billing rules.[19] The rules changed the billing for 23 laboratory tests that cover nearly two-thirds of all laboratory testing.[19]
Return on investment
The return on investment (ROI) metric is important to the POL. An example of ROI in action is when someone invests in a stock and gets 10% of the money back every year; the 10% would be the ROI from a purely financial standpoint. The formula for an ROI calculation is listed below:
- Simple ROI = Financial Gain/Total Investment
- Discounted ROI = Net Present Value of Benefits/Total Present Value of Costs[20]
The Simple ROI calculation is primarily used for short-term calculations related to an investment of one year or less, while the Discounted ROI is more accurate for long-term analysis and calculations.[20]
The factors included in the ROI calculation will vary, depending on the type of calculation. The Simple ROI, for example, only examines financial gain divided by total investment amount. The person using this formula would say, after gaining $110,000 by investing $100,000, their ROI is 110%.
Before performing either calculation, it is important to measure current performance and then measure performance again after the investment.
The POL example below uses the IT Economics Corporation ROI calculation formula[20]:
Simple ROI calculation for Fein and Douglas Associates' POL
Year 1
$100,000 benefit to the practice - $100,000 outlay of resources to establish the lab = $0 net savings divided by $100,000 outlay of resources, yielding a Simple ROI of 0%
Year 2
$400,000 benefit to the practice - $100,000 outlay of resources to maintain the laboratory = $300,000 net savings divided by $100,000 outlay of resources, yielding a Simple ROI of 300%
Year 3
$700,000 benefit to the practice - $100,000 outlay of resources to maintain the laboratory = $600,000 net savings divided by $100,000 outlay of resources, yielding a Simple ROI of 600%.
The Simple ROI calculation becomes less accurate as time goes on, because it does not take into account any discounting for the value of money or other assets impacted by time, such as equipment depreciation.
The Discounted ROI methodology takes into account a dollar received in 1988 is worth more than one received in 2008. It is more complicated than the Simple ROI, as it requires calculating the present value of costs and benefits, and it also requires knowing the organization discount rate. The organization CFO is typically the best person to contact for that information.
Note how taking the same laboratory and performing a Discounted ROI calculation shows a more accurate result, as seen below[20]:
Discounted ROI calculation for Fein and Douglas Associates' POL
For this example, the assumption of an average 6% discount rate was used.
Year 1
Present Value (PV) of Benefits: $100,000 received at the end of year 1 = $94,340
PV of Costs: $100,000 paid at the end of year 1 = $94,340
$94,340 PV of benefits to the practice - $94,340 PV of costs to establish the lab = $0 Net PV of benefits divided by $94,340 PV of costs, yielding a Discounted ROI of 0%
Note how the Simple ROI and the Discounted ROI calculation are the same during the first year due to the cost and benefits all occurring the same year and at the same time.
Year 2
Present Value (PV) of Benefits: $100,000 received at the end of year 1 = $94,340; PV of another $300,000 received at the end of year 2 = $267,000; Net PV of Benefits for year 2 thus = $361,340
PV of Costs: $100,000 paid at the end of year 2 = $94,340
$361,340 PV of benefits to the practice - $94,340 PV of costs to maintain the lab = $267,000 Net PV of benefits divided by $94,340 PV of costs, yielding a Discounted ROI of 283%
Note how the Simple ROI for year 2 was 300%, a full 17% greater than the discounted ROI.
Year 3
Present Value (PV) of Benefits: $100,000 received at the end of year 1 = $94,340; PV of another $300,000 received at the end of year 2 = $267,000; PV of another $300,000 received at the end of year 3 = $251,886; Net PV of Benefits for year 3 thus = $613,226
PV of Costs: $100,000 paid at the end of year 3 = $94,340
$613,226 PV of benefits to the practice - $94,340 PV of costs to maintain the lab = $518,886 Net PV of benefits divided by $94,340 PV of costs, yielding a Discounted ROI of 550%
Discounted ROI at the end of year 3 is 550%, not the 600% shown by the Simple ROI calculation. In this case the overage is 50% in year 3, as opposed to 17% in year 2. The Simple ROI calculation becomes less accurate as future years increase, which means that by year four, the overage would be greater than 50%.[20]
Managing data and test results
Data management in the physician office laboratory has six important aspects to it:
- Overall workflow
- Order entry
- Testing, including associated results and reports
- Quality control
- Integration with instruments and software
- Integration with external reference laboratory results
In any modern laboratory, the common way to integrate data and workflow across the enterprise is through a data management system.
Data management systems and test workflow
From a basic clinical and research laboratory perspective, there are two common data management systems to choose from: a laboratory information system (LIS) and a laboratory information management system (LIMS). Generally speaking, a LIS will be found more often in a clinical laboratory like the POL, whereas a LIMS will be more common in a research laboratory.[21]
The functionality is slightly different between the LIS and LIMS. The LIS tends to be more patient-centric, exhibiting features that focus on subjects and specimens, while the LIMS tends to be more group-centric, focusing on batches and samples. Both of these systems may have the ability to interface with a hospital information system (HIS), electronic health record (EHR), practice management system (PMS), or other types of systems commonly found in healthcare settings.
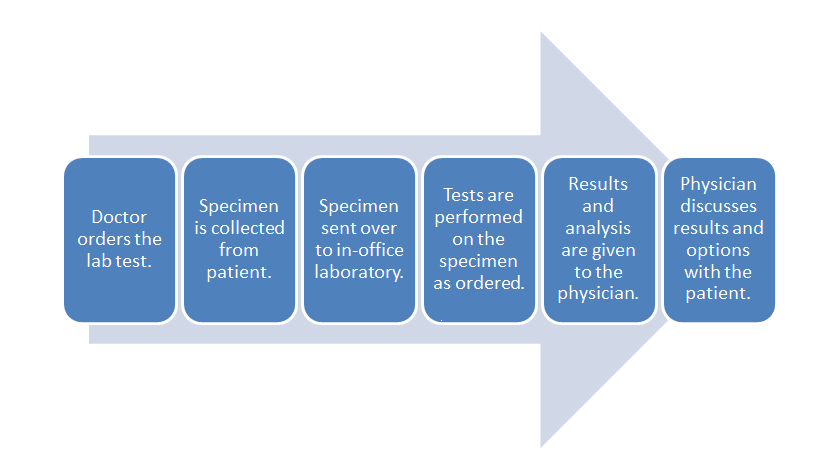
Overall, workflow was discussed in a previous section, but just to refresh the reader's mind, here is the chart again:
- POL workflow:
Notice the flow starts with the doctor ordering the test and ends with the discussion of options with the patient. POL workflow may vary depending upon specialty, but the steps identified in this guide are the most common. Each part of the workflow is important and dependent upon the other. Without a doctor order, it does not matter if the specimen is collected and sent to the lab. Without a specimen to test, the rest of this workflow is unable to proceed.
Since the first step is a doctor ordering the test, it is important to understand how the order entry component of a LIS operates. Each vendor will have a slightly different display, but the process is typically the same. The doctor enters the order for the test, often through a drop-down menu of tests in the patient's chart through the provider's EHR system. The LIS integrates to the EHR, allowing the laboratory staff to receive the order to collect the samples, perform the tests, and then transfer the results back into the EHR record for the physician to review, often in the form of a report. If the physician is not using an electronic system, then this process begins with a written or printed order and finishes with a written or printed report of the results for the patient file.
Many LIS vendors will custom configure the system with tests, including reference ranges for the POL as part of the purchase agreement. These interfaces will often have a drop-down menu as well, so that the person performing the test can select the test and compare the sample result to the reference range for that particular test.
Quality control
Aside from workflow, order entry, testing, and reporting, another important aspect of managing data is quality control (QC), which allows the handler of the data to ensure it still meets the definition of quality data. In healthcare informatics, quality data is defined as data that is clear, complete, relevant, timely, and accurate in presentation.[22]
Prior to the use of computers, the entire process of informatics involved paper records. In many cases, these records were handwritten, requiring personnel to read the handwriting of others in order to assess the quality of the data provided.[23] Resistance to using a computer for laboratory tasks typically comes from a belief that the old ways are better; however, in most cases the computer can greatly aid in the prevention of errors.[23]
LIS and LIMS vendors often include a variety of quality control functions in their software. QC tests can be run on specimens, quality control charts and reports can be created, proficiency testing functions can be implemented, and certificates of analysis (COAs) can be produced.[24]
It's worth noting, though, the use of computers and information management systems does not completely rule out error. For example, the drop-down menu still allows users to select either the wrong test or a test that looks for the same result but is less effective than another test.[23] These types of errors are more often than not controlled in a system where the laboratory personnel are knowledgeable about testing and are able to educate the ordering physician on such matters.[23]
Other business processes can benefit from quality control measures, such as the application of Lean Six Sigma — an approach to reducing waste and limiting defects in a process — to the laboratory. It is important to examine the needs of the laboratory to ensure appropriate quality control techniques are implemented.
Integration with instruments and software
Since workflow is the single most important consideration in the design of the LIS[23], it is important it allows instruments and other software to interface. These interfaces are generally done using standard communication processes and systems, as well as messaging formats, like Health Level 7 (HL7).[23]
While the majority of POLs do waived testing, and those simple tests don't often require advanced equipment, interfacing a data management system may not be a concern. But for those POLs that employ laboratory automation, the ability of the equipment to talk to the LIS is vital, often using HL7.
The types of information communicated between these systems include process control and status information for each device or analyzer, each specimen, specimen container, and container carrier, information and detailed data related to patients, orders, and results, and information related to specimen flow algorithms and automated decision making.[25]
A software interface between the LIS and the EHR is often referred to as a result interface, and it typically uses the HL7 messaging protocol and standard communication protocols like TCP/IP. These interfaces are not turn-key, however, requiring a comprehensive planning and implementation process.[26] After a successful implementation, the interface allows information from a completed test to be reported back to the EHR, where the physician can readily obtain a copy of the patient test report. It can also allow for billing batches and admission/discharge/transfer (ADT) reporting. These same interfaces can be used for communicating with other reference laboratories, in addition to the various hospital systems.[23]
Getting help with your POL
It is important those involved with the POL know where to get help when issues arise. Often, the first call goes to the vendor of the software, instruments, testing supplies, and other products and services the laboratory utilizes. If the vendor is unable to assist, consultants may prove to be a valuable asset to the POL. Consultants can help staff sort through regulatory compliance, financial planning, systems planning, and laboratory design. Consultants can also assist with filling the gap between a vendor support agreement and the need of the POL, if such a gap exists in the contract.
Another route for support with your POL may be a professional organization or trade association. The American College of Physicians, for example, provides numerous printable resources to practices. The American Society for Clinical Laboratory Science offers professional development courses, educational courses, and certification help to its members.
A directory of consultants, organizations, and other tools is available as an addendum to this guide.
Conclusion
POLs have existed in various forms since the beginning of medicine. With accountable care organizations (ACO), patient-centered medical homes, direct-to-consumer laboratory testing, and telemedicine, an increased opportunity exists for laboratory medicine to be added to practices. Laboratory medicine influences 70% of all medical decisions, and the POL allows the physician to get results faster than having to send out for testing at a reference laboratory.
The decision to operate (or continue operating) a POL is an important one not to be entered into lightly. Owners must consider regulatory compliance, business processes, technology choices, and economic considerations. However, while a daunting task, ultimately choosing to operate a POL may prove to be a rewarding experience.
References
- ↑ New York State Department of Health, Wadsworth Center (2014). "Physician Office Laboratory Evaluation Program (POLEP)". http://www.wadsworth.org/labcert/polep/. Retrieved 14 May 2014.
- ↑ 2.0 2.1 UnitedHealthcare Oxford (1 July 2012). "Oxford's in-office laboratory testing and procedures list" (PDF). https://www.oxhp.com/secure/policy/oxfords_in_office_laboratory_testing_and_procedures_list.pdf. Retrieved 14 May 2014.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 3.14 3.15 Berger, D. (July 1999). "A brief history of medical diagnosis and the birth of the clinical laboratory: Part 1—Ancient times through the 19th century" (PDF). Medical Laboratory Observer 31 (7): 28–30, 32, 34–40. http://www.academia.dk/Blog/wp-content/uploads/KlinLab-Hist/LabHistory1.pdf. Retrieved 14 May 2014.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 Berger, D. (August 1999). "A brief history of medical diagnosis and the birth of the clinical laboratory: Part 2—Laboratory science and professional certification in the 20th century" (PDF). Medical Laboratory Observer 31 (8): 32–34, 36, 38. http://www.academia.dk/Blog/wp-content/uploads/KlinLab-Hist/LabHistory2.pdf. Retrieved 14 May 2014.
- ↑ 5.0 5.1 5.2 Berger, D. (October 1999). "A brief history of medical diagnosis and the birth of the clinical laboratory: Part 3—Medicare, government regulation and competency certification" (PDF). Medical Laboratory Observer 31 (10): 40–42, 44. http://www.academia.dk/Blog/wp-content/uploads/KlinLab-Hist/LabHistory3.pdf. Retrieved 14 May 2014.
- ↑ 6.0 6.1 6.2 6.3 Kusserow, R. P. (March 1989). Quality assurance in physician office labs. OAI-0588-00330. U.S. Department of Health and Human Services, Office of Analysis and Inspections. https://oig.hhs.gov/oei/reports/oai-05-88-00330.pdf. Retrieved 14 May 2014.
- ↑ 7.0 7.1 Bachman, A. (2004). "Prosperity in the POL" (PDF). ADVANCE for Administrators of the Laboratory 13 (12): 66. http://laboratory-manager.advanceweb.com/Article/Prosperity-in-the-POL.aspx. Retrieved 14 May 2014.
- ↑ Centers for Medicare and Medicaid Services, Division of Laboratory Services (December 2013). "Laboratories by type of facility" (PDF). https://www.cms.gov/Regulations-and-Guidance/Legislation/CLIA/downloads/factype.pdf. Retrieved 14 May 2014.
- ↑ Centers for Medicare and Medicaid Services, Division of Laboratory Services (December 2013). "Enrollment, CLIA exempt states, and certification of accreditation by organization" (PDF). http://www.cms.gov/Regulations-and-Guidance/Legislation/CLIA/Downloads/statupda.pdf. Retrieved 14 May 2014.
- ↑ 10.00 10.01 10.02 10.03 10.04 10.05 10.06 10.07 10.08 10.09 10.10 Centers for Disease Control and Prevention (31 May 2013). "Clinical Laboratory Improvement Amendments (CLIA): Test complexities". http://wwwn.cdc.gov/clia/Resources/TestComplexities.aspx. Retrieved 14 May 2014.
- ↑ American Academy of Family Physicians (2014). "Physician office laboratory (POL) director duties". http://www.aafp.org/practice-management/regulatory/clia/pol-director-duties.html. Retrieved 14 May 2014.
- ↑ Olea, S. (2012). "CLIA required personnel qualifications". http://www.jointcommission.org/assets/1/18/CLIA_required_personnel_qualifications.pdf. Retrieved 14 May 2014.
- ↑ American Academy of Family Physicians (2014). "Personnel requirements". http://www.aafp.org/practice-management/regulatory/clia/personnel.html. Retrieved 14 May 2014.
- ↑ 14.0 14.1 14.2 U.S. Department of Health and Human Services (3 February 2014). "HHS strengthens patients' right to access lab test reports". http://www.hhs.gov/news/press/2014pres/02/20140203a.html.
- ↑ 15.0 15.1 Conn, J. (3 February 2014). "HHS issues rule granting patients direct access to lab test results". Modern Healthcare. http://www.modernhealthcare.com/article/20140203/NEWS/302039958. Retrieved 14 May 2014.
- ↑ 16.0 16.1 16.2 Clinical Laboratory Coalition (2012). "Protect access to laboratory services for Medicare beneficiaries" (PDF). http://www.aab.org/images/aab/SGR%20Fix%202012%20Talking%20Points.pdf. Retrieved 14 May 2014.
- ↑ 17.0 17.1 Hughes, D.; Cammarata, B. (16 January 2014). "Clinical labs under ACA: Challenge and opportunity". Law360. http://www.law360.com/articles/500623/clinical-labs-under-aca-challenge-and-opportunity. Retrieved 14 May 2014.
- ↑ HIMSS (1 April 2014). "After passage by Congress, President signs SGR "Doc Fix" & ICD-10 delay". http://www.himss.org/News/NewsDetail.aspx?ItemNumber=28914. Retrieved 14 May 2014.
- ↑ 19.0 19.1 Baker, B.; McKenzie, C. (December 2013). "Recent CMS lab test standards simplify billing rules". ACP Observer. http://www.acpinternist.org/archives/2003/12/baker.htm. Retrieved 14 May 2014.
- ↑ 20.0 20.1 20.2 20.3 20.4 IT Economics Corporation (2010). "Computing the ROI for IT projects and other investments". http://iteconcorp.com/ROICalc.html. Retrieved 14 May 2014.
- ↑ Friedman, B. (4 November 2008). "LIS vs. LIMS: It's time to blend the two types of lab information systems". Lab Soft News. http://labsoftnews.typepad.com/lab_soft_news/2008/11/liss-vs-limss-its-time-to-consider-merging-the-two-types-of-systems.html. Retrieved 14 May 2014.
- ↑ World Health Organization, Regional Office for the Western Pacific (2003). "Data quality: A guide for developing countries". World Health Organization. http://www.wpro.who.int/publications/docs/Improving_Data_Quality.pdf. Retrieved 14 May 2014.
- ↑ 23.0 23.1 23.2 23.3 23.4 23.5 23.6 Sinard, J. (2006). Practical pathology informatics: Demystifying informatics for the practicing anatomic pathologist. Springer Science+Business Media. ISBN 9780387280585. http://www.springer.com/medicine/pathology/book/978-0-387-28057-8.
- ↑ Hull, C.; Wray, B.; Winslow, F.; Villicich, M. (2011). "Tracking and controlling everything that affects quality is the key to a quality management system". Combinatorial Chemistry & High Throughput Screening 14 (9): 772–780. http://www.ncbi.nlm.nih.gov/pubmed/21631414. Retrieved 14 May 2014.
- ↑ Health Level Seven International (2011). "HL7 version 2.7 standard: Chapter 13 - Clinical laboratory automation". http://www.hl7.org/implement/standards/product_brief.cfm?product_id=203. Retrieved 14 May 2014.
- ↑ Kasoff, J. (February 2012). "Connecting your LIS and EHR". Medical Laboratory Observer. http://www.mlo-online.com/articles/201202/connecting-your-lis-and-ehr.php. Retrieved 14 May 2014.
Appendix
Table 1. Tests Granted Waived Status under CLIA (This list includes updates from Change Request 8439.)
| CPT Code(s) | Test Name | Manufacturer | Use |
|---|---|---|---|
| 81002 | Dipstick or tablet reagent urinalysis – non-automated for bilirubin, glucose, hemoglobin, ketone, leukocytes, nitrite, pH, protein, specific gravity, and urobilinogen | Various | Screening of urine to monitor/diagnose various diseases/conditions, such as diabetes, the state of the kidney or urinary tract, and urinary tract infections |
| 81025 | Urine pregnancy tests by visual color comparison | Various | Diagnosis of pregnancy |
| 82270 82272 (Contact your Medicare carrier for claims instructions.) |
Fecal occult blood | Various | Detection of blood in feces from whatever cause, benign or malignant (colorectal cancer screening) |
| 82962 | Blood glucose by glucose monitoring devices cleared by the FDA for home use | Various | Monitoring of blood glucose levels |
| 83026 | Hemoglobin by copper sulfate – non-automated | Various | Monitors hemoglobin level in blood |
| 84830 | Ovulation tests by visual color comparison for human luteinizing hormone | Various | Detection of ovulation (optimal for conception) |
| 85013 | Blood count; spun microhematocrit | Various | Screen for anemia |
| 85651 | Erythrocyte sedimentation rate – non-automated | Various | Nonspecific screening test for inflammatory activity, increased for majority of infections, and most cases of carcinoma and leukemia |
(Recreated from http://www.cms.gov/Regulations-and-Guidance/Legislation/CLIA/downloads/waivetbl.pdf)
Addendum
As part of the "Getting help with your POL" section, an addendum to this white paper is included, containing information about conferences, consultants, organizations, and published materials that could potentially help those operating and working in a physician office laboratory.
You can find that content on this page.