Difference between revisions of "Main Page/Featured article of the week/2023"
Shawndouglas (talk | contribs) (Added last week's article of the week) |
Shawndouglas (talk | contribs) m (Updates) |
||
| (50 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{ombox | {{ombox | ||
| type = notice | | type = notice | ||
| text = If you're looking for other "Article of the Week" archives: [[Main Page/Featured article of the week/2014|2014]] - [[Main Page/Featured article of the week/2015|2015]] - [[Main Page/Featured article of the week/2016|2016]] - [[Main Page/Featured article of the week/2017|2017]] - [[Main Page/Featured article of the week/2018|2018]] - [[Main Page/Featured article of the week/2019|2019]] - [[Main Page/Featured article of the week/2020|2020]] - [[Main Page/Featured article of the week/2021|2021]] - [[Main Page/Featured article of the week/2022|2022]] - 2023 | | text = If you're looking for other "Article of the Week" archives: [[Main Page/Featured article of the week/2014|2014]] - [[Main Page/Featured article of the week/2015|2015]] - [[Main Page/Featured article of the week/2016|2016]] - [[Main Page/Featured article of the week/2017|2017]] - [[Main Page/Featured article of the week/2018|2018]] - [[Main Page/Featured article of the week/2019|2019]] - [[Main Page/Featured article of the week/2020|2020]] - [[Main Page/Featured article of the week/2021|2021]] - [[Main Page/Featured article of the week/2022|2022]] - 2023 - [[Main Page/Featured article of the week/2024|2024]] | ||
}} | }} | ||
| Line 17: | Line 17: | ||
<!-- Below this line begin pasting previous news --> | <!-- Below this line begin pasting previous news --> | ||
<h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: January 16–22:</h2> | <h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: December 25–31:</h2> | ||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig1 Larobina Tomography23 9-5.png|240px]]</div> | |||
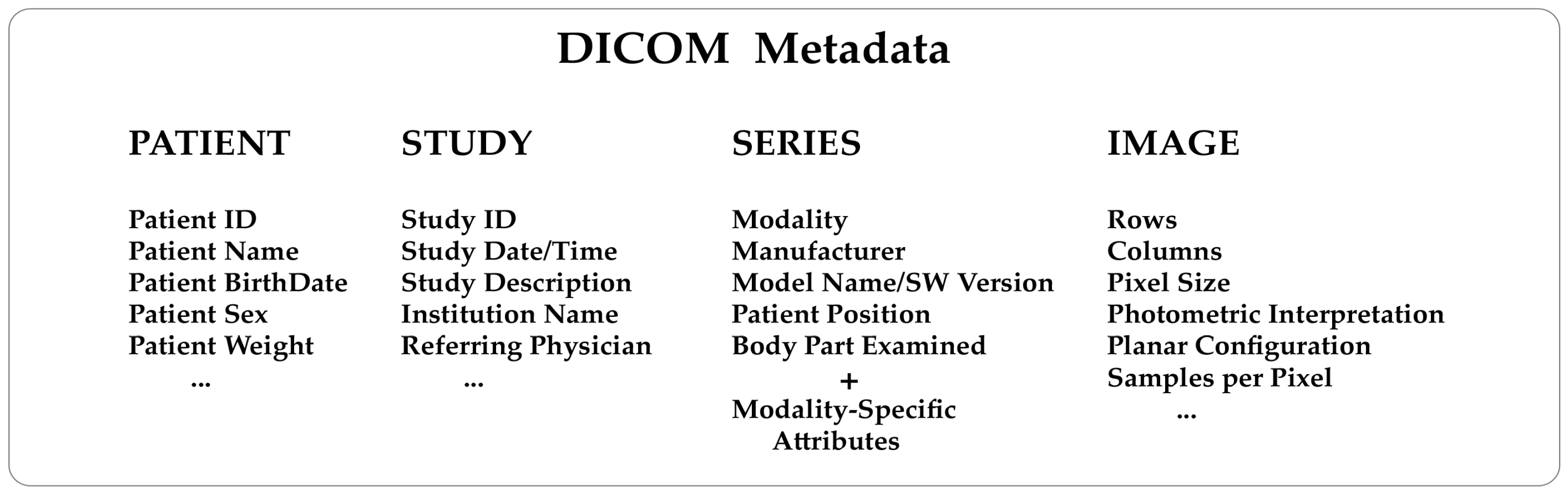
'''"[[Journal:Thirty years of the DICOM standard|Thirty years of the DICOM standard]]"''' | |||
[[DICOM|Digital Imaging and Communications in Medicine]] (DICOM) is an international standard that defines a format for storing [[Medical imaging|medical images]] and a protocol to enable and facilitate data communication among medical imaging systems. The DICOM standard has been instrumental in transforming the medical imaging world over the last three decades. Its adoption has been a significant experience for manufacturers, healthcare users, and research scientists. In this review, 30 years after introducing the standard, we discuss the innovation, advantages, and limitations of adopting DICOM and its possible future directions ... ('''[[Journal:Thirty years of the DICOM standard|Full article...]]''')<br /> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: December 18–24:</h2> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig2 Xu BMCMedEd23 23.png|240px]]</div> | |||
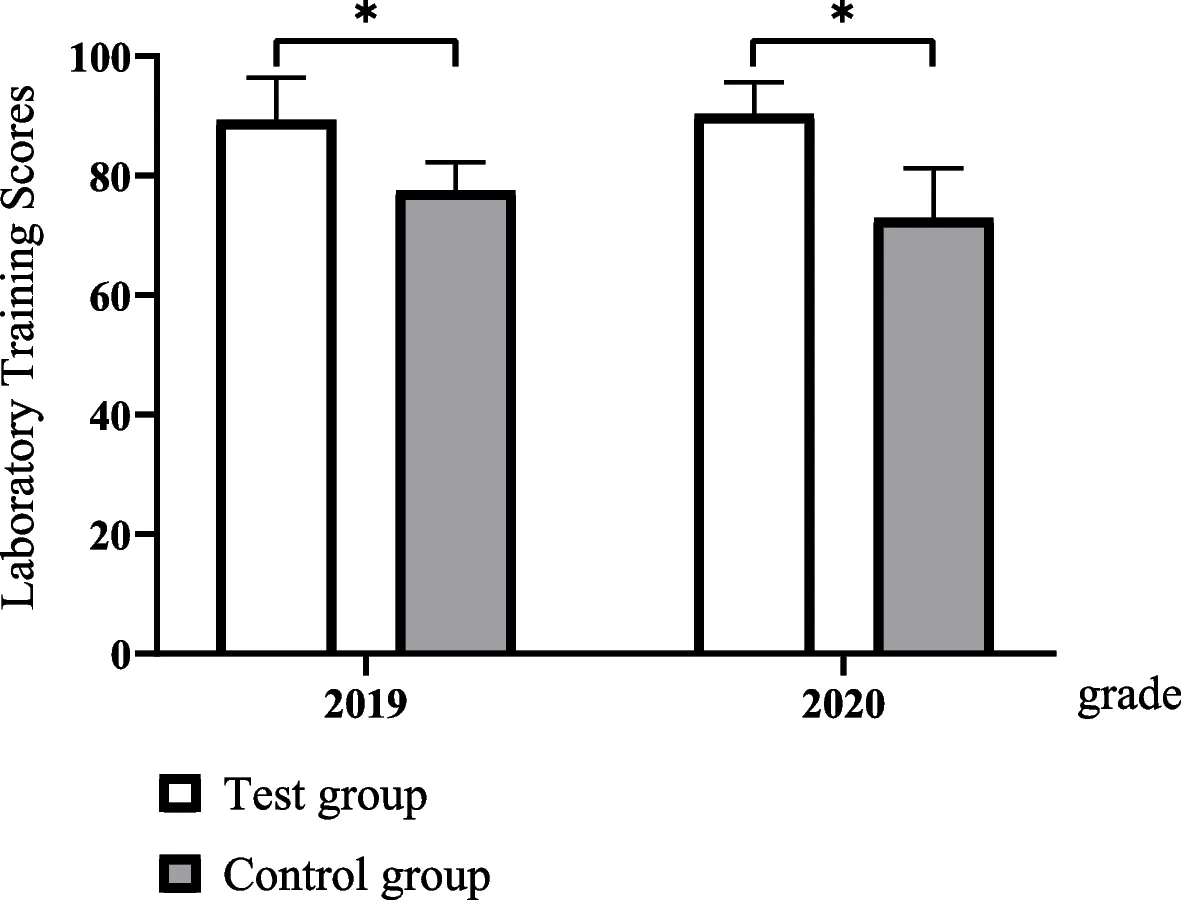
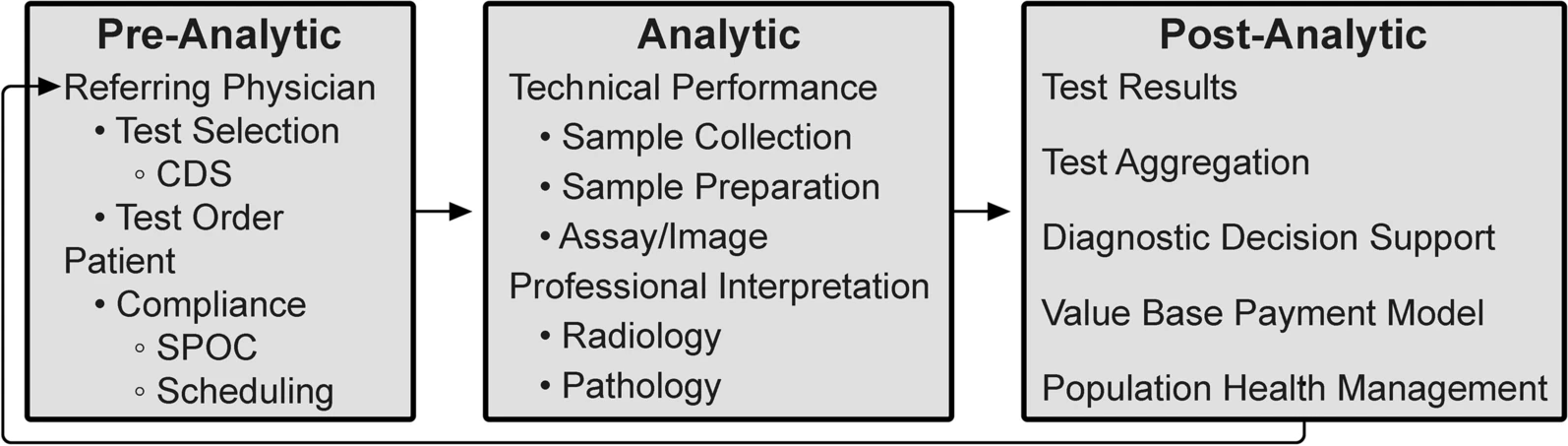
'''"[[Journal:Evaluating the effectiveness of a new student-centred laboratory training strategy in clinical biochemistry teaching|Evaluating the effectiveness of a new student-centred laboratory training strategy in clinical biochemistry teaching]]"''' | |||
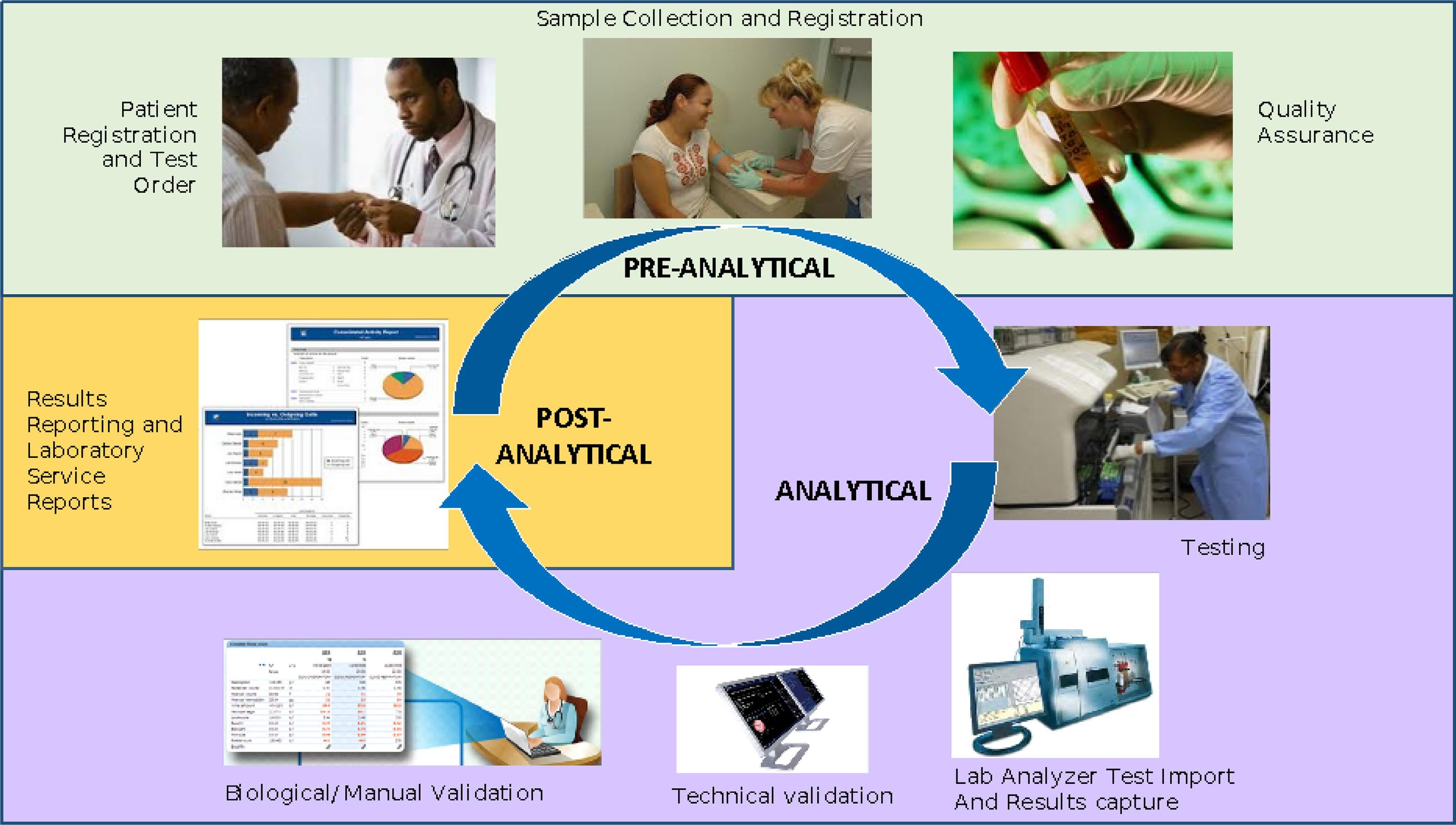
The error-proneness in the pre-analytical and post-analytical stages is higher than that in the analytical stage of the total [[laboratory]] testing process. However, pre-analytical and post-analytical [[Quality (business)|quality]] management has not received enough attention in [[Clinical laboratory|medical laboratory]] education and tests in clinical [[biochemistry]] courses. Clinical biochemistry teaching programs aim to improve students’ awareness of and ability to use quality management practices according to the [[International Organization for Standardization]]'s [[ISO 15189]] requirements ... ('''[[Journal:Evaluating the effectiveness of a new student-centred laboratory training strategy in clinical biochemistry teaching|Full article...]]''')<br /> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: December 11–17:</h2> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig1 Tamura SciTechAdvMatMeth2023 3-1.jpeg|240px]]</div> | |||
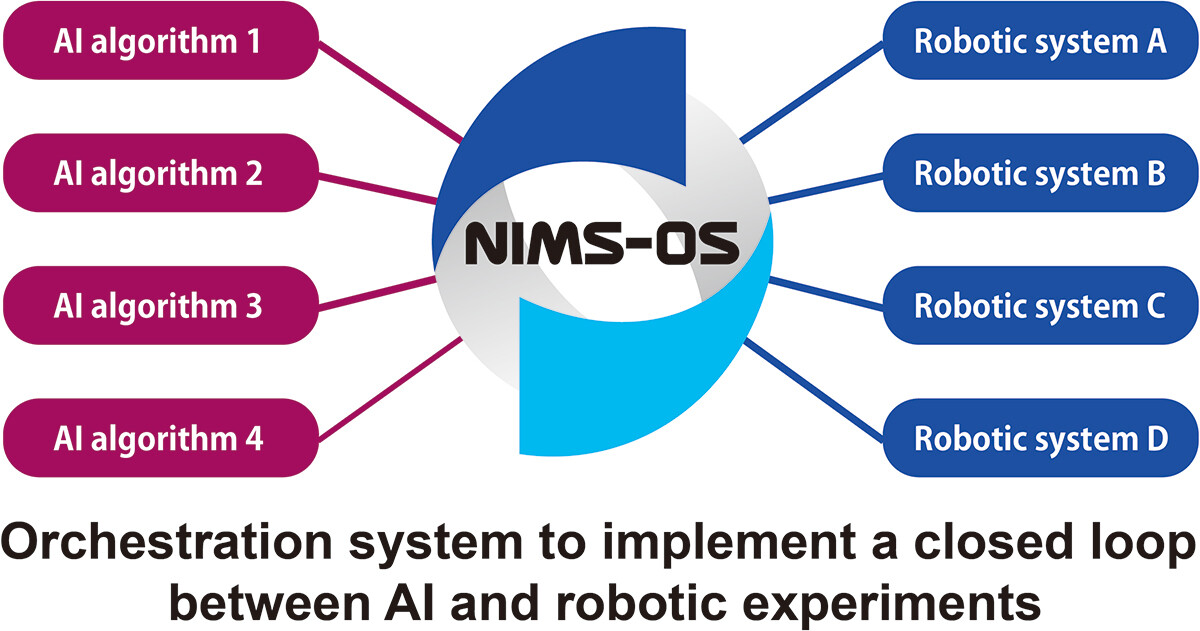
'''"[[Journal:NIMS-OS: An automation software to implement a closed loop between artificial intelligence and robotic experiments in materials science|NIMS-OS: An automation software to implement a closed loop between artificial intelligence and robotic experiments in materials science]]"''' | |||
NIMS-OS (NIMS Orchestration System) is a [[Python (programming language)|Python]] library created to realize a closed loop of [[Laboratory automation|robotic]] experiments and [[artificial intelligence]] (AI) without human intervention for automated [[Materials science|materials exploration]]. It uses various combinations of modules to operate autonomously. Each module acts as an AI for materials exploration or a controller for a robotic experiments. As AI techniques, Optimization Tools for PHYSics Based on Bayesian Optimization (PHYSBO), BoundLess Objective-free eXploration (BLOX), phase diagram construction (PDC), and random exploration (RE) methods can be used. Moreover, a system called NIMS Automated Robotic Electrochemical Experiments (NAREE) is available as a set of robotic experimental equipment ... ('''[[Journal:NIMS-OS: An automation software to implement a closed loop between artificial intelligence and robotic experiments in materials science|Full article...]]''')<br /> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: December 04–10:</h2> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig1 Lehmann Sensors23 23-1.png|240px]]</div> | |||
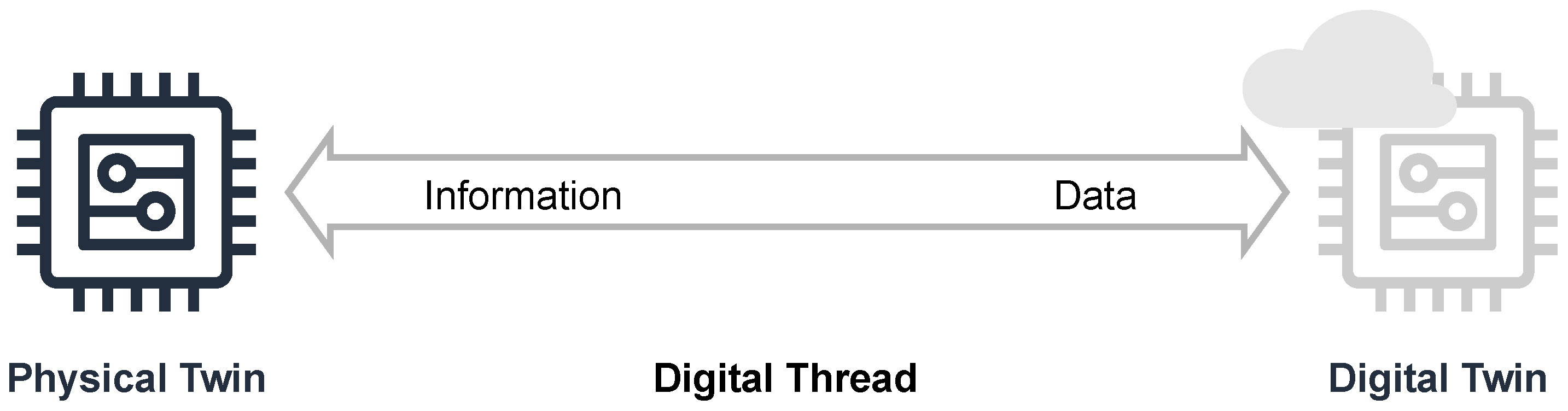
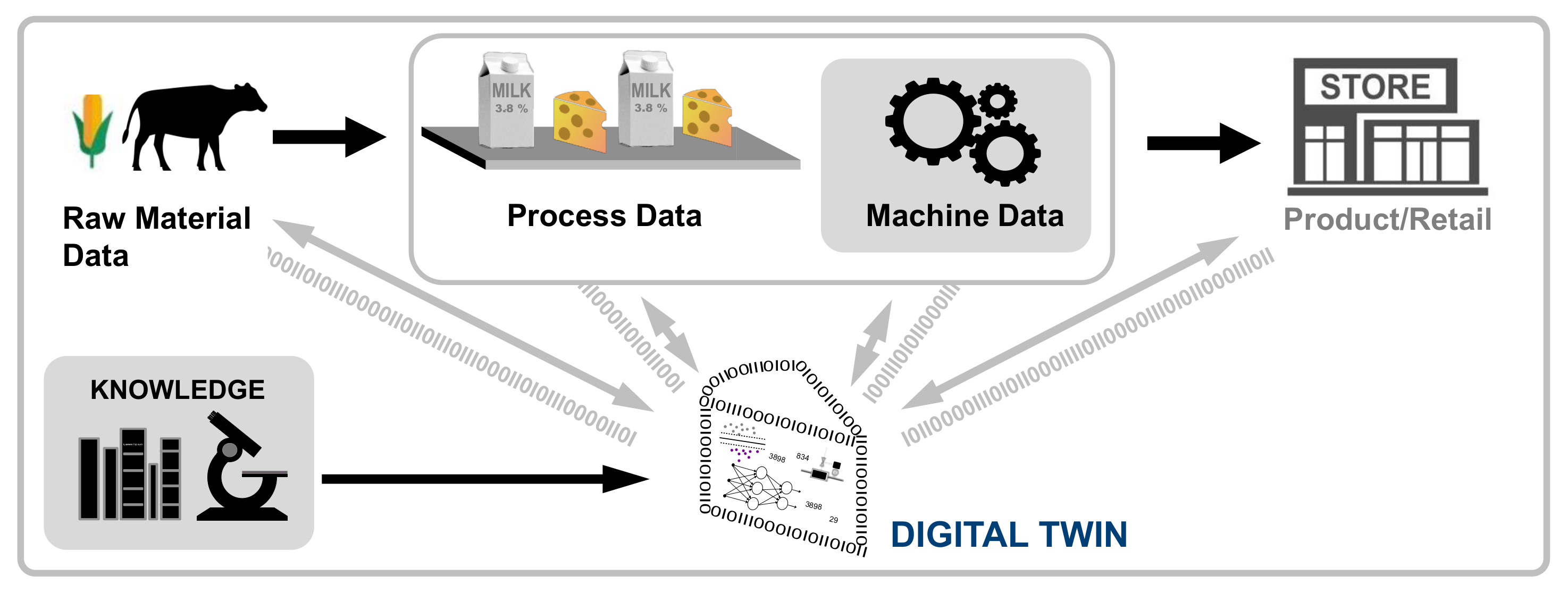
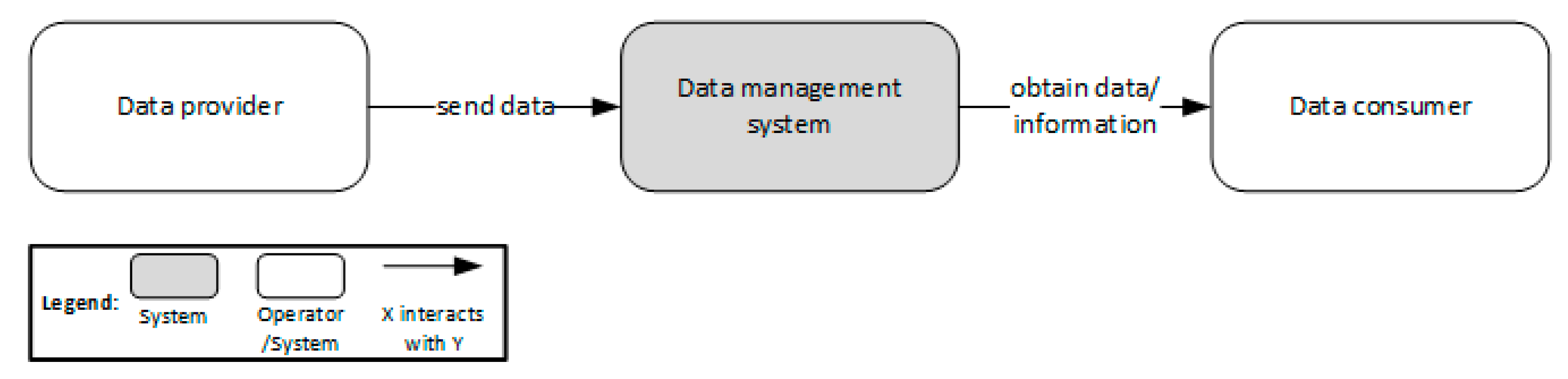
'''"[[Journal:Establishing reliable research data management by integrating measurement devices utilizing intelligent digital twins|Establishing reliable research data management by integrating measurement devices utilizing intelligent digital twins]]"''' | |||
One of the main topics within [[research]] activities is the [[Information management|management of research data]]. Large amounts of data acquired by heterogeneous scientific devices, sensor systems, measuring equipment, and experimental setups have to be processed and ideally managed by [[Journal:The FAIR Guiding Principles for scientific data management and stewardship|FAIR]] (findable, accessible, interoperable, and reusable) data management approaches in order to preserve their intrinsic value to researchers throughout the entire data lifecycle. The symbiosis of heterogeneous measuring devices, FAIR principles, and [[digital twin]] technologies is considered to be ideally suited to realize the foundation of reliable, sustainable, and open research data management ... ('''[[Journal:Establishing reliable research data management by integrating measurement devices utilizing intelligent digital twins|Full article...]]''')<br /> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: November 27–December 03:</h2> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig1 Ishizuki SciTechAdvMatMeth2023 3-1.jpeg|240px]]</div> | |||
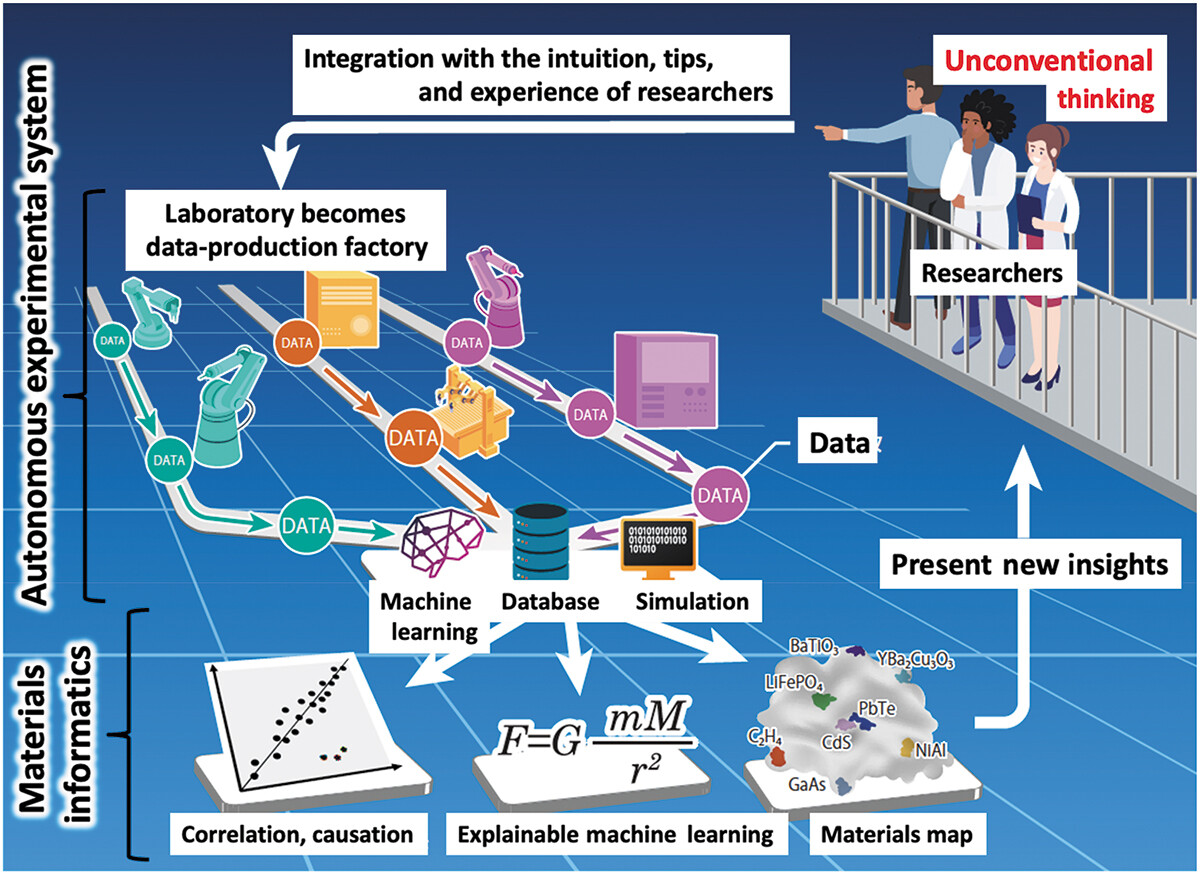
'''"[[Journal:Autonomous experimental systems in materials science|Autonomous experimental systems in materials science]]"''' | |||
The emergence of [[Laboratory automation|autonomous experimental systems]] (AESs) integrating [[machine learning]] (ML) and robots is ushering in a paradigm shift in [[materials science]]. Using computer algorithms and robots to decide and perform all experimental steps, these systems require no human intervention. A current direction focuses on discovering unexpected materials and theories with unconventional [[research]] approaches. This article reviews the latest achievements and discusses the impact of AESs, which will fundamentally change the way we understand research. Moreover, as AESs continue to develop, the need to think about the role of human researchers becomes more pressing ... ('''[[Journal:Autonomous experimental systems in materials science|Full article...]]''')<br /> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: November 20–26:</h2> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig2 Ayaz Healthcare23 11-12.png|240px]]</div> | |||
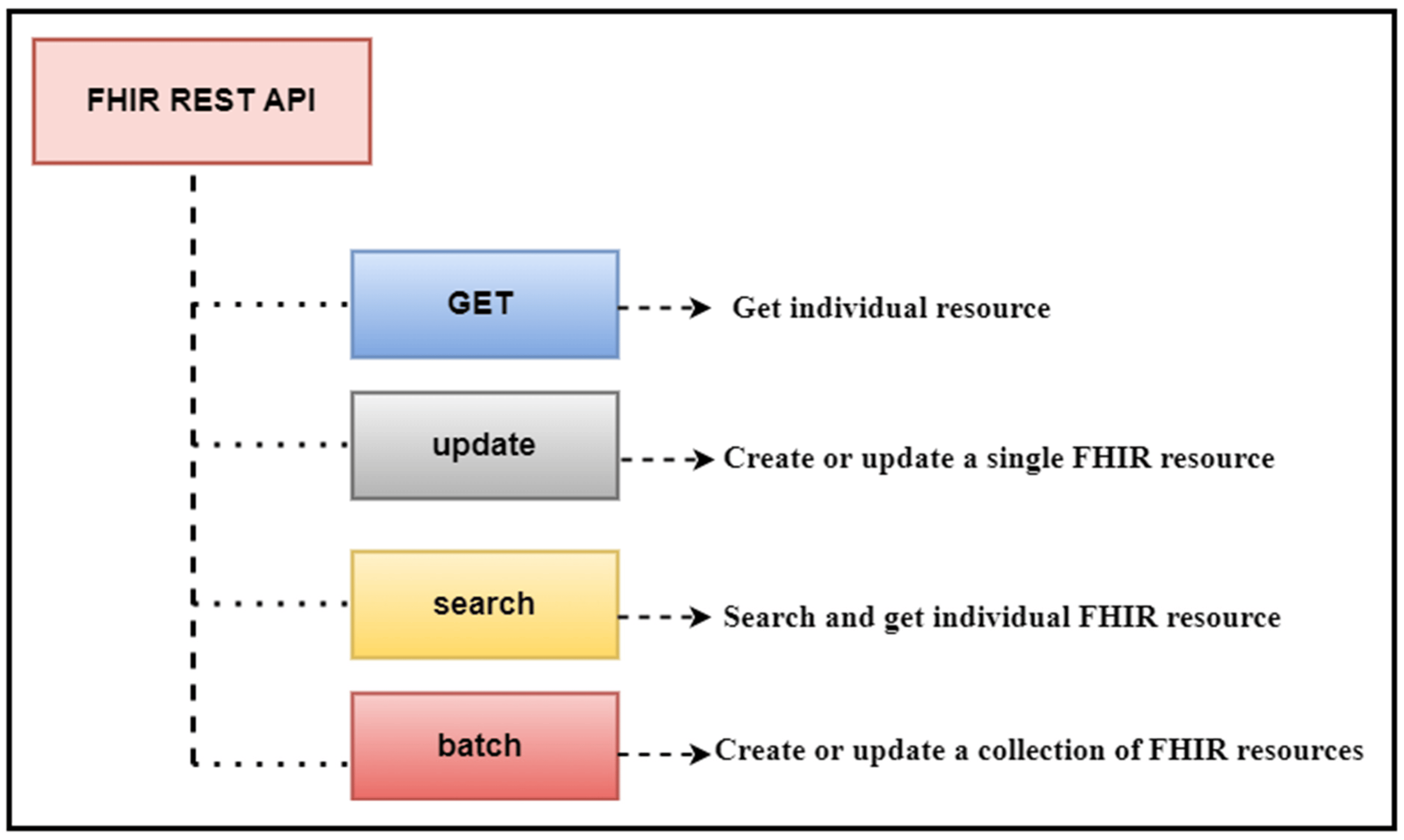
'''"[[Journal:Transforming healthcare analytics with FHIR: A framework for standardizing and analyzing clinical data|Transforming healthcare analytics with FHIR: A framework for standardizing and analyzing clinical data]]"''' | |||
In this study, we discuss our contribution to building a [[Data analysis|data analytic]] framework that supports clinical statistics and analysis by leveraging a scalable standards-based data model named [[Fast Healthcare Interoperability Resources]] (FHIR). We developed an intelligent algorithm that is used to facilitate the clinical data analytics process on FHIR-based data. We designed several [[workflow]]s for patient clinical data used in two [[hospital information system]]s (HISs), namely patient registration systems (PRSs) and [[laboratory information system]]s (LIS). These workflows exploit various FHIR [[application programming interface]]s (API) to facilitate patient-centered and cohort-based interactive analyses. We developed a FHIR database implementation that utilizes FHIR APIs and a range of operations to facilitate descriptive data analytics (DDA) and patient cohort selection ... ('''[[Journal:Transforming healthcare analytics with FHIR: A framework for standardizing and analyzing clinical data|Full article...]]''')<br /> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: November 13–19:</h2> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig1 Mencacci FrontCellInfectMicro2023 13.jpg|240px]]</div> | |||
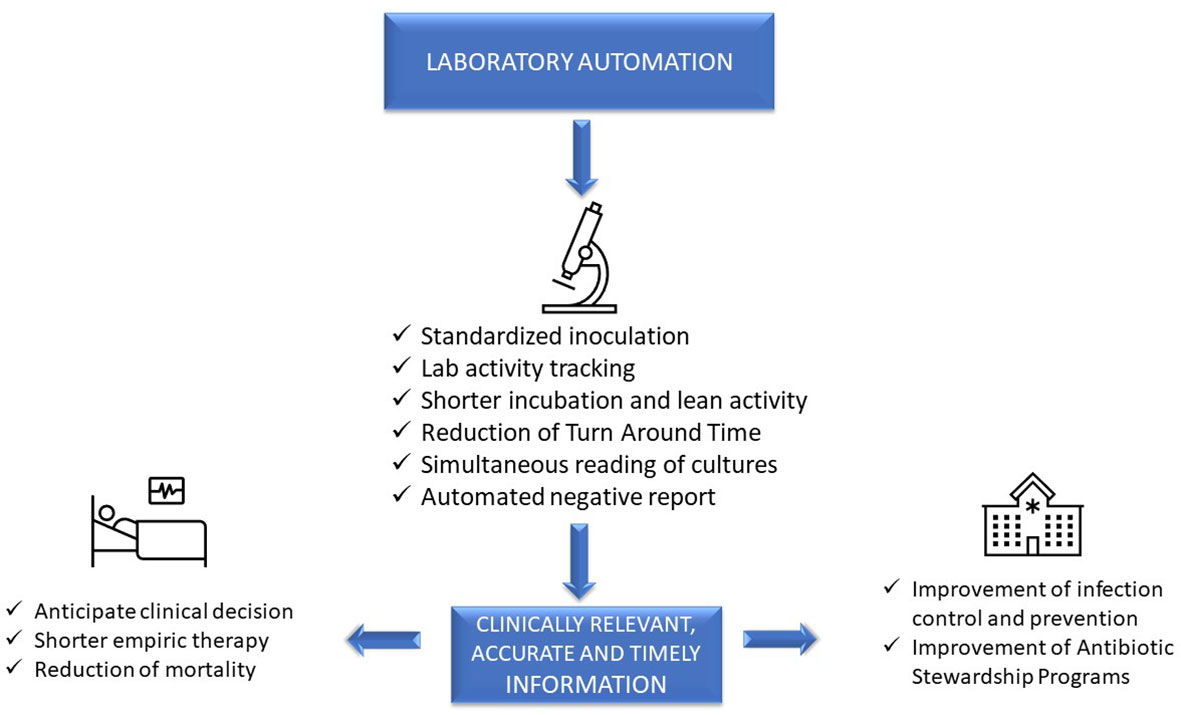
'''"[[Journal:Laboratory automation, informatics, and artificial intelligence: Current and future perspectives in clinical microbiology|Laboratory automation, informatics, and artificial intelligence: Current and future perspectives in clinical microbiology]]"''' | |||
[[Clinical laboratory|Clinical diagnostic laboratories]] produce one product—[[information]]—and for this to be valuable, the information must be clinically relevant, accurate, and timely. Although diagnostic information can clearly improve patient outcomes and decrease healthcare costs, technological challenges and [[laboratory]] [[workflow]] practices affect the timeliness and clinical value of diagnostics. This article will examine how prioritizing laboratory practices in a patient-oriented approach can be used to optimize technology advances for improved patient care. ... ('''[[Journal:Laboratory automation, informatics, and artificial intelligence: Current and future perspectives in clinical microbiology|Full article...]]''')<br /> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: November 06–12:</h2> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig2 Zheng Electronics23 12-8.png|240px]]</div> | |||
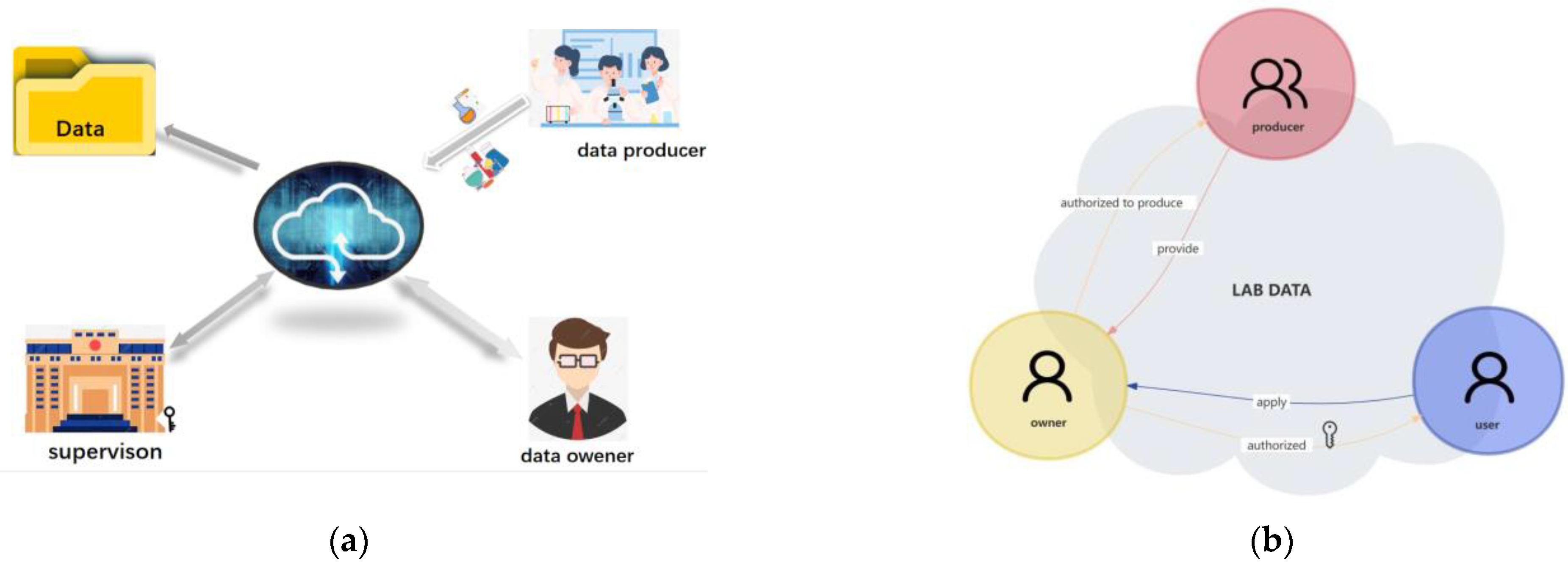
'''"[[Journal:Registered data-centered lab management system based on data ownership security architecture|Registered data-centered lab management system based on data ownership security architecture]]"''' | |||
University and college [[Laboratory|laboratories]] are important places to train professional and technical personnel. Various regulatory departments in colleges and universities still rely on traditional laboratory management in [[research]] projects, which are prone to problems such as untimely [[information]] and data transmission. The present study aimed to propose a new method to solve the problem of data islands, explicit ownership, conditional sharing, [[Information security|data security]], and efficiency during laboratory [[Information management|data management]]. Hence, this study aimed to develop a data-centered lab management system that enhances the security of laboratory data management and allows the data owners of the labs to control [[data sharing]] with other users ... ('''[[Journal:Registered data-centered lab management system based on data ownership security architecture|Full article...]]''')<br /> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: October 30–November 05:</h2> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:GA Hauschild iScience2022 25-12.jpg|240px]]</div> | |||
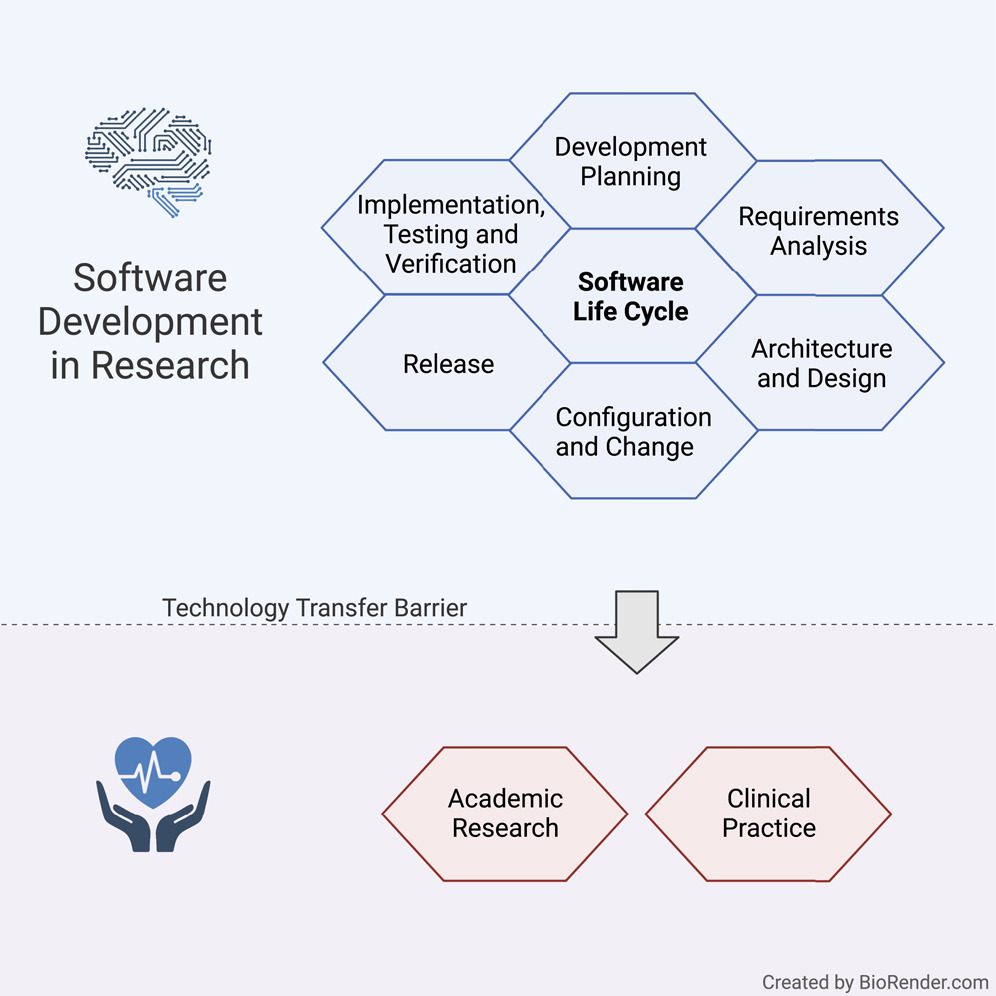
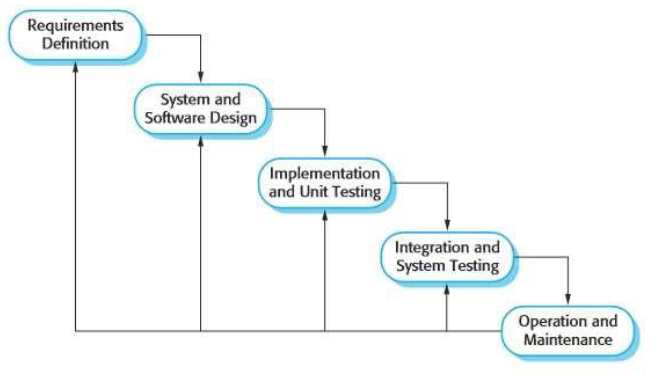
'''"[[Journal:Guideline for software life cycle in health informatics|Guideline for software life cycle in health informatics]]"''' | |||
The long-lasting trend of [[medical informatics]] is to adapt novel technologies in the medical context. In particular, incorporating [[artificial intelligence]] (AI) to support clinical decision-making can significantly improve monitoring, diagnostics, and prognostics for the patient’s and medic’s sake. However, obstacles hinder a timely technology transfer from the medical research setting to the actual clinical setting. Due to the pressure for novelty in the [[research]] context, projects rarely implement [[Quality (business)|quality]] standards. Here, we propose a guideline for academic software life cycle (SLC) processes tailored to the needs and capabilities of research organizations ... ('''[[Journal:Guideline for software life cycle in health informatics|Full article...]]''')<br /> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: October 23–29:</h2> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:GA Hauschild iScience2022 25-12.jpg|240px]]</div> | |||
'''"[[Journal:Guideline for software life cycle in health informatics|Guideline for software life cycle in health informatics]]"''' | |||
The long-lasting trend of [[medical informatics]] is to adapt novel technologies in the medical context. In particular, incorporating [[artificial intelligence]] (AI) to support clinical decision-making can significantly improve monitoring, diagnostics, and prognostics for the patient’s and medic’s sake. However, obstacles hinder a timely technology transfer from the medical research setting to the actual clinical setting. Due to the pressure for novelty in the [[research]] context, projects rarely implement [[Quality (business)|quality]] standards. Here, we propose a guideline for academic software life cycle (SLC) processes tailored to the needs and capabilities of research organizations ... ('''[[Journal:Guideline for software life cycle in health informatics|Full article...]]''')<br /> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: October 16–22:</h2> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig4 Shen BMCMedInfoDecMak23 23.png|240px]]</div> | |||
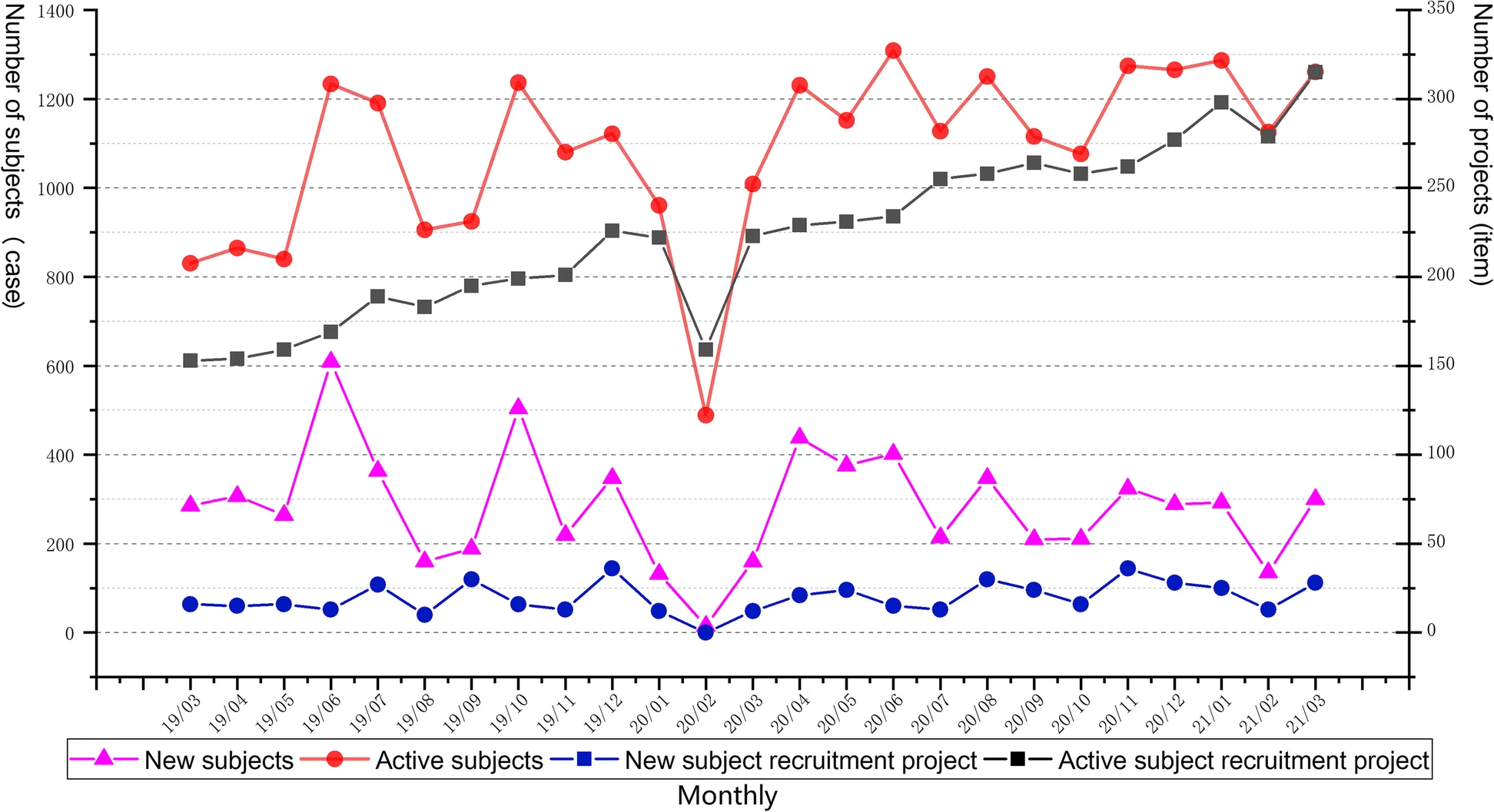
'''"[[Journal:Development of an integrated and comprehensive clinical trial process management system|Development of an integrated and comprehensive clinical trial process management system]]"''' | |||
The process of initiating and completing clinical drug trials in [[hospital]] settings is highly complex, with numerous institutional, technical, and record-keeping barriers. In this study, we independently developed an integrated [[clinical trial management system]] (CTMS) designed to comprehensively optimize the process management of clinical trials. The CTMS includes system development methods, efficient integration with external business systems, terminology, and standardization protocols, as well as [[Information security|data security]] and [[Information privacy|privacy]] protection ... ('''[[Journal:Development of an integrated and comprehensive clinical trial process management system|Full article...]]''')<br /> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: October 09–15:</h2> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig1 Pablo JofPathInfo2023 14.jpg|240px]]</div> | |||
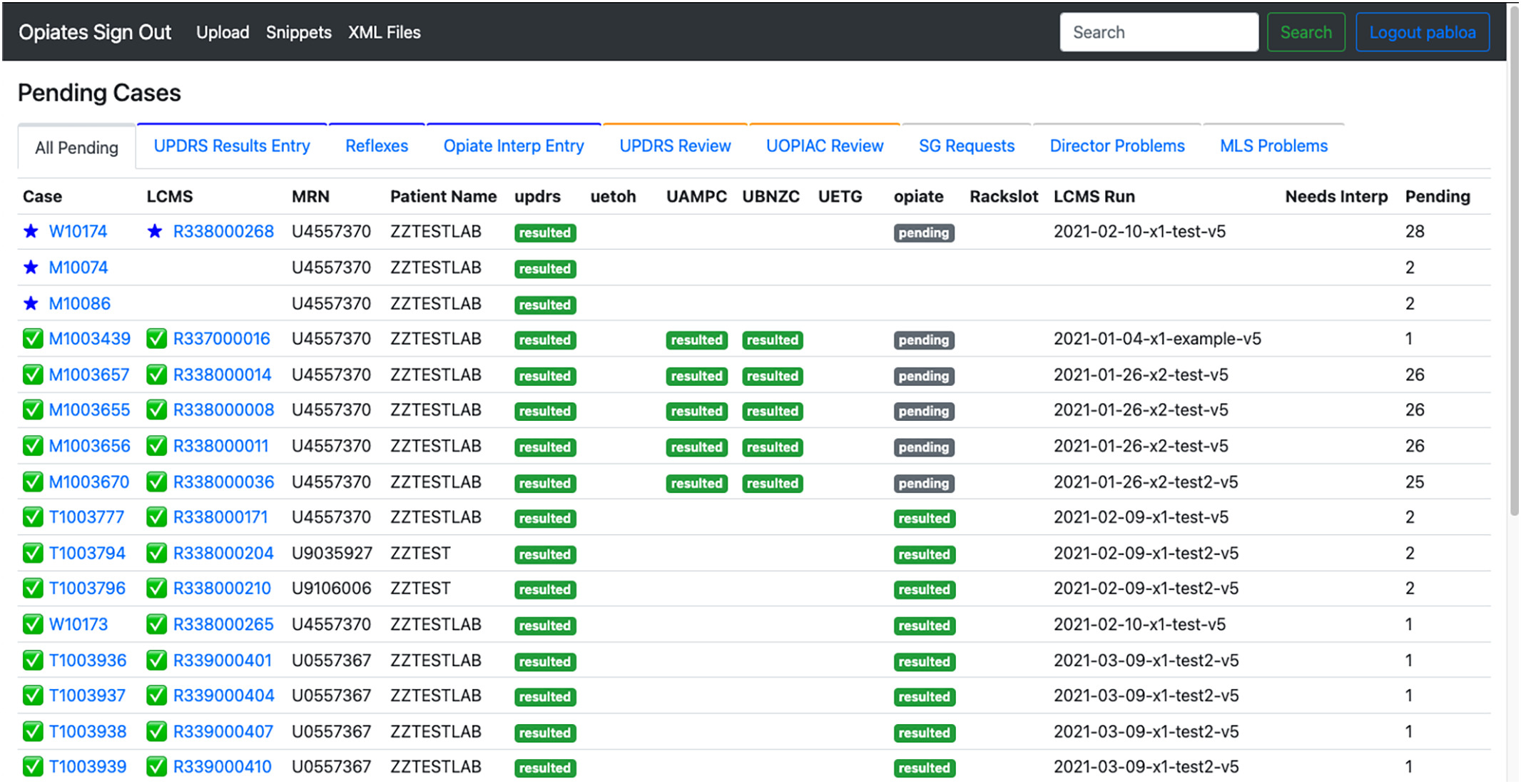
'''"[[Journal:A web application to support the coordination of reflexive, interpretative toxicology testing|A web application to support the coordination of reflexive, interpretative toxicology testing]]"''' | |||
[[Reflex test|Reflexive laboratory testing]] [[workflow]]s can improve the assessment of patients receiving pain medications chronically, but complex workflows requiring [[Pathology|pathologist]] input and interpretation may not be well-supported by traditional [[laboratory information system]]s (LISs). In this work, we describe the development of a web application that improves the efficiency of pathologists and [[laboratory]] staff in delivering actionable [[toxicology]] results. Before designing the application, we set out to understand the entire workflow, including the laboratory workflow and pathologist review ... ('''[[Journal:A web application to support the coordination of reflexive, interpretative toxicology testing|Full article...]]''')<br /> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: October 02–08:</h2> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig1.1 Davis FrontBioinfo2022 40.jpg|240px]]</div> | |||
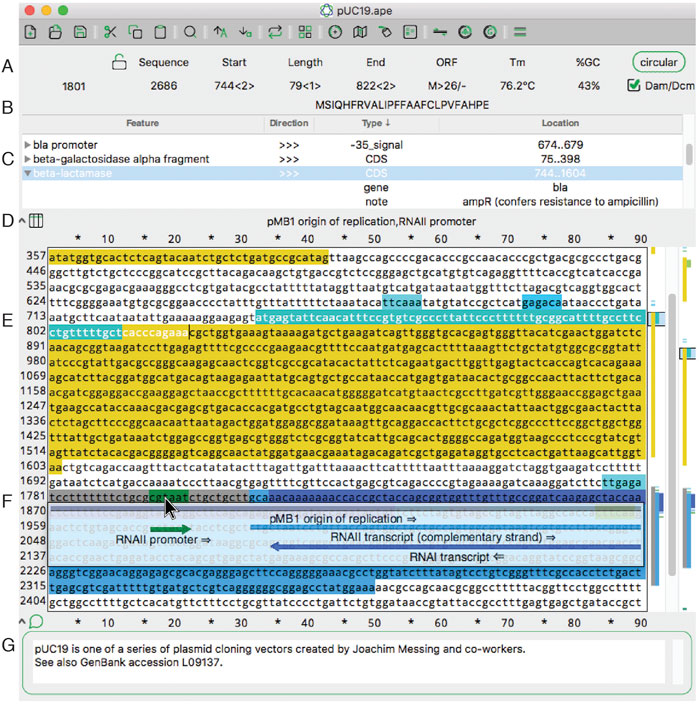
'''"[[Journal:ApE, A Plasmid Editor: A freely available DNA manipulation and visualization program|ApE, A Plasmid Editor: A freely available DNA manipulation and visualization program]]"''' | |||
A Plasmid Editor (ApE) is a free, multi-platform application for [[Data visualization|visualizing]], designing, and presenting biologically relevant [[DNA sequencing|DNA sequences]]. ApE provides a flexible framework for annotating a sequence manually or using a user-defined library of features. ApE can be used in designing [[plasmid]]s and other constructs via ''in silico'' simulation of cloning methods such as [[polymerase chain reaction]] (PCR), Gibson assembly, restriction-ligation assembly, and Golden Gate assembly. In addition, ApE provides a platform for creating visually appealing linear and circular plasmid maps. It is available for Mac, PC, and Linux-based platforms ... ('''[[Journal:ApE, A Plasmid Editor: A freely available DNA manipulation and visualization program|Full article...]]''')<br /> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: September 25–October 01:</h2> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig1 He IntJofMedInfo2023 170.jpg|240px]]</div> | |||
'''"[[Journal:Development and national scale implementation of an open-source electronic laboratory information system (OpenELIS) in Côte d’Ivoire: Sustainability lessons from the first 13 years|Development and national scale implementation of an open-source electronic laboratory information system (OpenELIS) in Côte d’Ivoire: Sustainability lessons from the first 13 years]]"''' | |||
Côte d'Ivoire has a tiered [[public health laboratory]] system of nine [[Reference laboratory|reference laboratories]], 77 [[Laboratory|laboratories]] at regional and general [[hospital]]s, and 100 laboratories among 1,486 district health centers. Prior to 2009, nearly all of these laboratories used paper registers and reports to collect and report laboratory data to clinicians and national disease monitoring programs. Since 2009 the Ministry of Health (MOH) in Côte d'Ivoire has sought to implement a comprehensive set of activities aimed at strengthening the laboratory system. One of these activities is the sustainable development, expansion, and technical support of an open-source electronic [[laboratory information system]] (LIS) called [[OpenELIS]], with the long-term goal of Ivorian technical support and managerial sustainment of the system ... ('''[[Journal:Development and national scale implementation of an open-source electronic laboratory information system (OpenELIS) in Côte d’Ivoire: Sustainability lessons from the first 13 years|Full article...]]''')<br /> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: September 18–24:</h2> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig3 Boobier JofChemInfoModel2023 63-10.png|240px]]</div> | |||
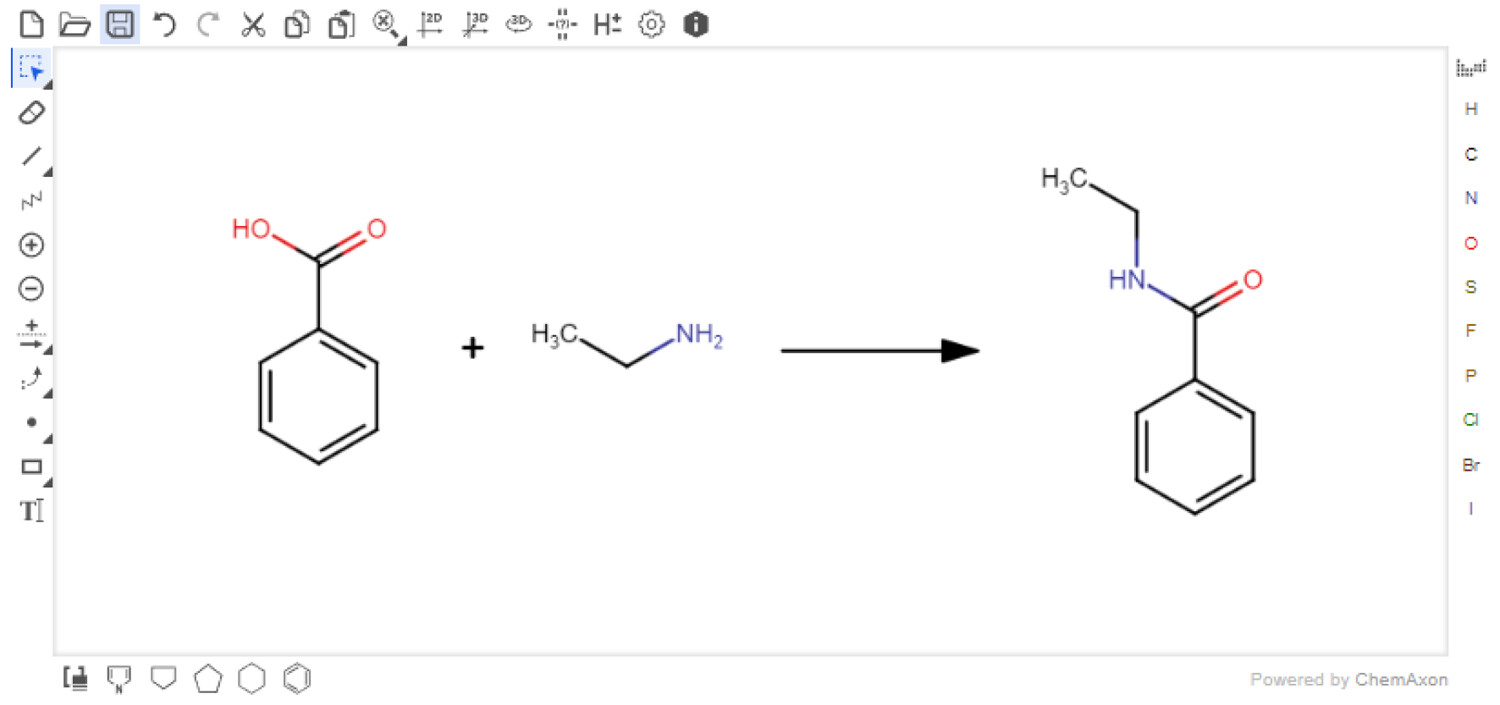
'''"[[Journal:AI4Green: An open-source ELN for green and sustainable chemistry|AI4Green: An open-source ELN for green and sustainable chemistry]]"''' | |||
This paper presents the [[Free and open-source software|free and open-source]], web-based [[electronic laboratory notebook]] (ELN) [[AI4Green]], which combines features such as data archiving, collaboration tools, and green and sustainability metrics for organic [[chemistry]]. AI4Green offers the core functionality of an ELN, namely, the ability to store reactions securely and [[Data sharing|share]] them among different members of a research team. As users plan their reactions and record them in the ELN, green and sustainable chemistry is encouraged by automatically calculating green metrics and color-coding hazards, solvents, and reaction conditions. The interface links a database constructed from data extracted from PubChem, enabling the automatic collation of [[information]] for reactions. The application’s design facilitates the development of auxiliary sustainability applications, such as our Solvent Guide module. As more reaction data are captured, subsequent work will focus on providing “intelligent” sustainability suggestions to the user. ('''[[Journal:AI4Green: An open-source ELN for green and sustainable chemistry|Full article...]]''')<br /> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: September 11–17:</h2> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig1 Ifriza Matrix23 13-1.png|240px]]</div> | |||
'''"[[Journal:The modeling of laboratory information systems in higher education based on enterprise architecture planning (EAP) for optimizing monitoring and equipment maintenance|The modeling of laboratory information systems in higher education based on enterprise architecture planning (EAP) for optimizing monitoring and equipment maintenance]]"''' | |||
The [[laboratory]] is a place to conduct scientific [[research]], experiments, measurements, or scientific training. Fakultas Matematika dan Ilmu Pengetahuan Alam, Universitas Negeri Semarang (FMIPA UNNES) has several laboratories distributed in each department to support student lectures. Through the implementation of practicum in the laboratory, students are expected to be able to find a concept, as well as foster scientific attitudes and critical thinking skills. Good laboratory management is expected to be able to utilize laboratory resources effectively and efficiently. [[Scientific instrument|Laboratory equipment]] must be ensured to function properly and be ready to be used for practicum. To support this, it is necessary to [[wikipedia:Condition monitoring|monitor the condition]] of the equipment and immediately repair the equipment if any damage is found. The current obstacle is monitoring tool repairs manually, so there are shortcomings such as poor documentation and equipment conditions that cannot be monitored online ... ('''[[Journal:The modeling of laboratory information systems in higher education based on enterprise architecture planning (EAP) for optimizing monitoring and equipment maintenance|Full article...]]''')<br /> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: September 04–10:</h2> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig1 Tziakou AccredQualAss23 28-3.png|240px]]</div> | |||
'''"[[Journal:Identifying risk management challenges in laboratories|Identifying risk management challenges in laboratories]]"''' | |||
Over the years, [[risk management]] has gained significant importance in [[Laboratory|laboratories]] of every kind. The safety of workers, the [[Accuracy and precision|accuracy]] and reliability of laboratory results, issues of financial sustainability, and protection of the environment play an important role in decision-making in both industry and service-based labs. In order for a laboratory to be considered reliable and safe, and therefore competitive, it is recommended to comply with the requirements of international standards and other [[Regulatory compliance|regulatory documents]], as well as use tools and risk management procedures. In this paper, [[information]] is summarized concerning the terms “risk” and “risk management,” which are then approached through the latest [[International Organization for Standardization]] (ISO) standard [[ISO 9000|ISO 9001]], [[ISO/IEC 17025]], and [[ISO 14000|ISO 14001]] standards ... ('''[[Journal:Identifying risk management challenges in laboratories|Full article...]]''')<br /> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: August 28–September 03:</h2> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig1 Nambiar BigDataCogComp22 6-4.png|240px]]</div> | |||
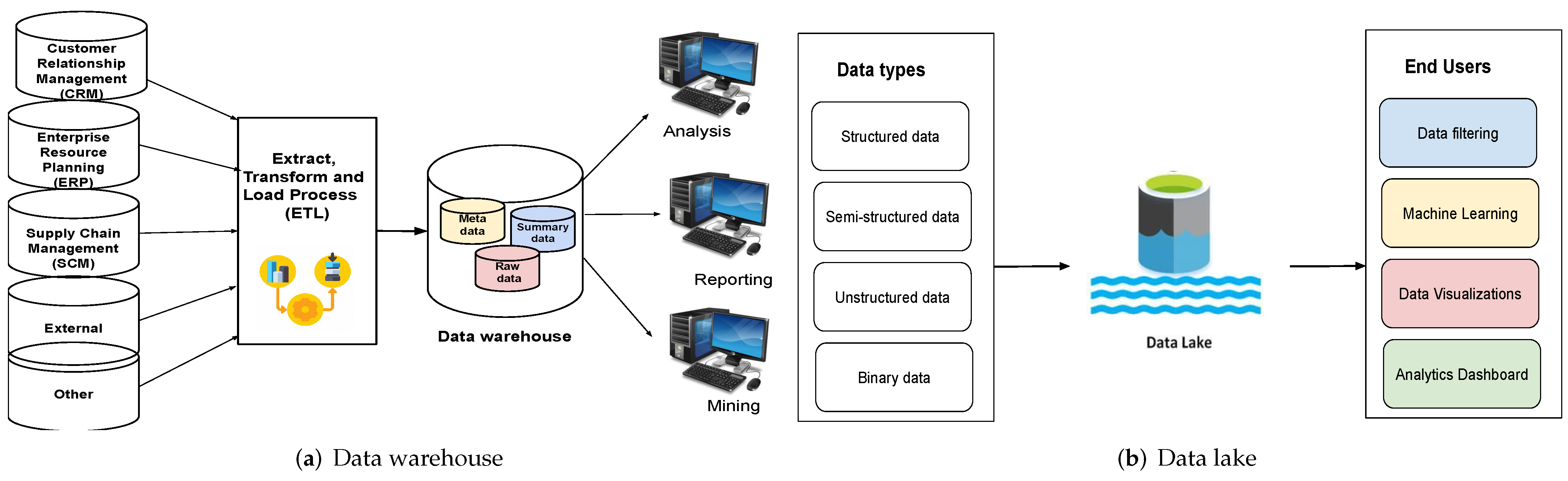
'''"[[Journal:An overview of data warehouse and data lake in modern enterprise data management|An overview of data warehouse and data lake in modern enterprise data management]]"''' | |||
Data is the lifeblood of any organization. In today’s world, organizations recognize the vital role of data in modern [[business intelligence]] systems for making meaningful decisions and staying competitive in the field. Efficient and optimal data analytics provides a competitive edge to its performance and services. Major organizations generate, collect, and process vast amounts of data, falling under the category of "big data." [[Information management|Managing]] and [[Data analysis|analyzing]] the sheer volume and variety of big data is a cumbersome process. At the same time, proper utilization of the vast collection of an organization’s [[information]] can generate meaningful insights into business tactics. In this regard, two of the more popular data management systems in the area of big data analytics—the [[data warehouse]] and [[data lake]]—act as platforms to accumulate the big data generated and used by organizations ... ('''[[Journal:An overview of data warehouse and data lake in modern enterprise data management|Full article...]]''')<br /> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: August 21–27:</h2> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig1 Kelly DataSciJourn22 21.png|240px]]</div> | |||
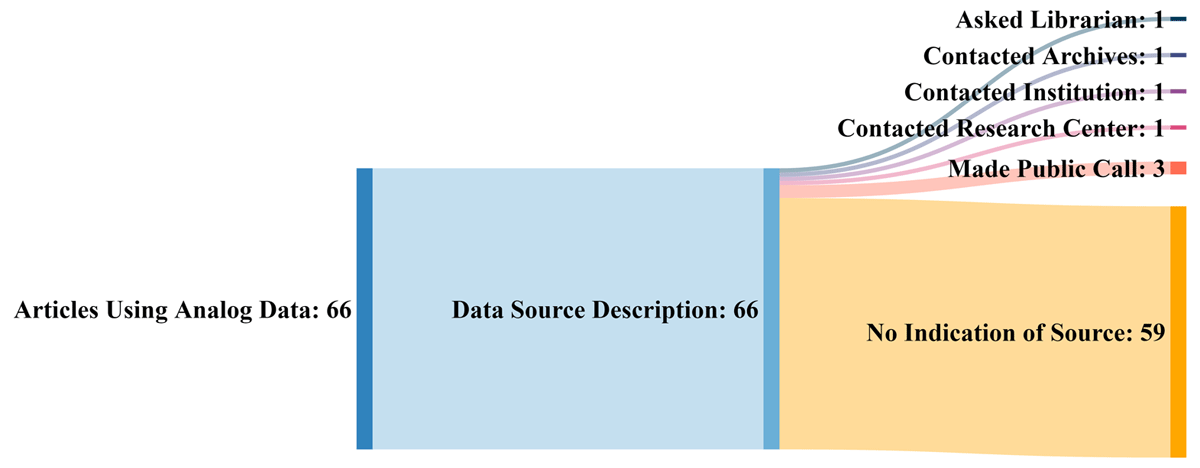
'''"[[Journal:A critical literature review of historic scientific analog data: Uses, successes, and challenges|A critical literature review of historic scientific analog data: Uses, successes, and challenges]]"''' | |||
For years, scientists in fields from climate change to biodiversity to hydrology have used older data to address contemporary issues. Since the 1960s, researchers, recognizing the value of this data, have expressed concern about its [[Information management|management]] and potential for loss. No widespread solutions have emerged to address the myriad issues around its storage, access, and findability. This paper summarizes observations and concerns of researchers in various disciplines who have articulated problems associated with analog data and highlights examples of projects that have used historical data. The authors also examined selected papers to discover how researchers located historical data and how they used it. While many researchers are not producing huge amounts of analog data today, there are still large volumes of it that are at risk. To address this concern, the authors recommend the development of best practices for managing historic data ... ('''[[Journal:A critical literature review of historic scientific analog data: Uses, successes, and challenges|Full article...]]''')<br /> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: August 14–20:</h2> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:GA1 Frede Processes23 11-1.png|240px]]</div> | |||
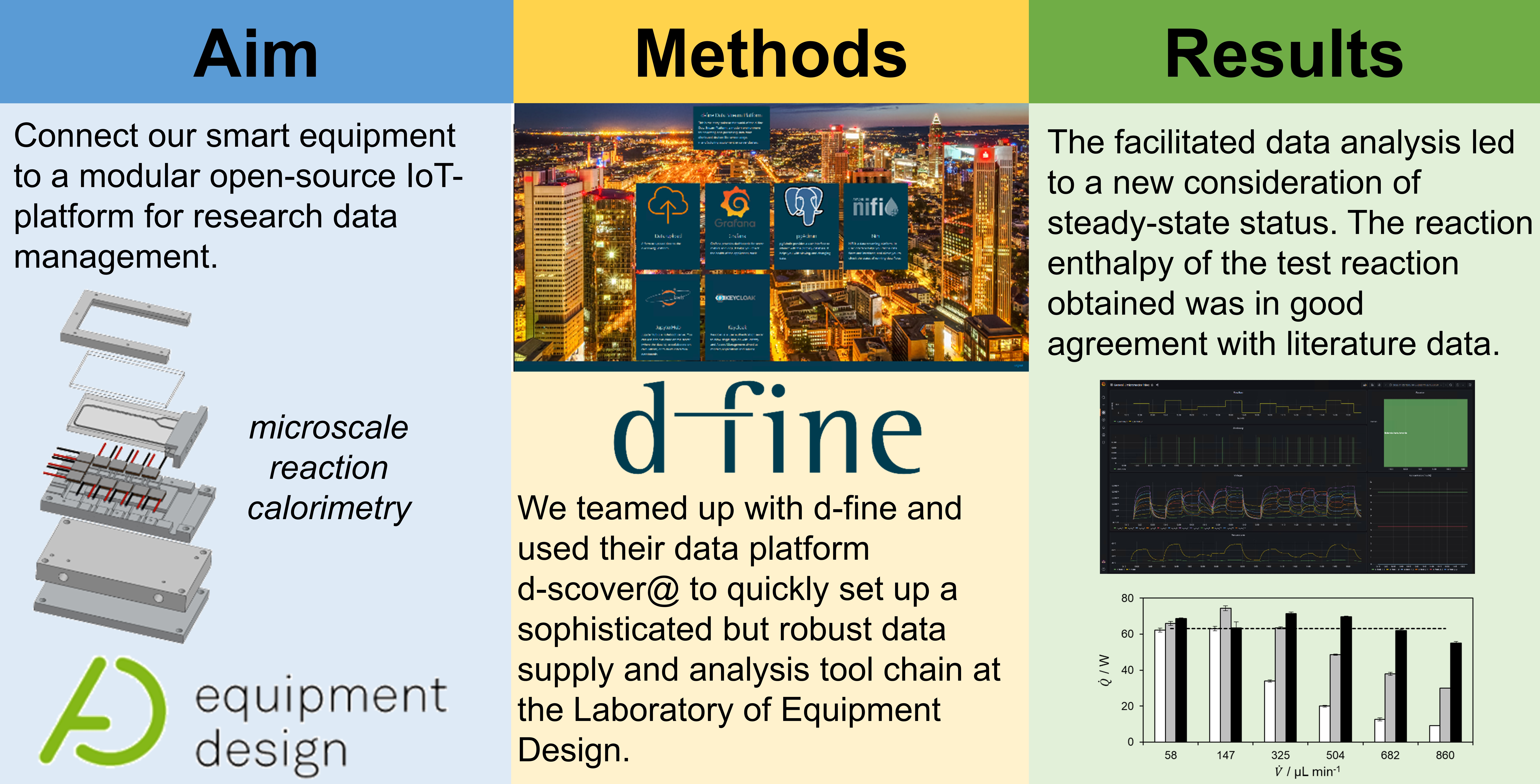
'''"[[Journal:Data management of microscale reaction calorimeter using a modular open-source IoT platform|Data management of microscale reaction calorimeter using a modular open-source IoT platform]]"''' | |||
Unifying research data collection methods and capturing data streams in an organized and standardized manner are becoming increasingly important in [[Laboratory|laboratories]] as digital processes and [[Laboratory automation|automation]] progressively shape the laboratory [[workflow]]s. In this context, the [[internet of things]] (IoT) not only offers the opportunity to minimize time-consuming and repetitive tasks by delegating them to machines, but it also supports scientists in curating data. As a contribution to the establishment of IoT tools in academic research laboratories, a microscale reaction [[calorimeter]] is exemplarily connected to a modular open-source IoT-platform. The microscale calorimeter’s process data is streamed to the data platform for storage and [[Data analysis|analysis]]. Advantages of the platform from academia’s point of view are presented ... ('''[[Journal:Data management of microscale reaction calorimeter using a modular open-source IoT platform|Full article...]]''')<br /> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: August 07–13:</h2> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig2 Beauchamp InsightsImaging2022 14.png|240px]]</div> | |||
'''"[[Journal:Integrative diagnostics: The time is now—a report from the International Society for Strategic Studies in Radiology|Integrative diagnostics: The time is now—a report from the International Society for Strategic Studies in Radiology]]"''' | |||
Enormous recent progress in [[Medical test|diagnostic testing]] can enable more accurate [[Medical diagnosis|diagnosis]] and improved clinical outcomes. Yet these tests are increasingly challenging and frustrating; the volume and diversity of results may overwhelm the diagnostic acumen of even the most dedicated and experienced clinician. Because they are gathered and processed within the “silo” of each diagnostic discipline, diagnostic data are fragmented, and the [[electronic health record]] (EHR) does little to synthesize new and existing data into usable [[information]]. Therefore, despite great promise, diagnoses may still be incorrect, delayed, or never made. Integrative diagnostics represents a vision for the future, wherein diagnostic data, together with clinical data from the EHR, are aggregated and contextualized by [[Informatics (academic field)|informatics]] tools to direct clinical action ... ('''[[Journal:Integrative diagnostics: The time is now—a report from the International Society for Strategic Studies in Radiology|Full article...]]''')<br /> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: July 31–August 06:</h2> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig1 Gonzales PLOSComBio22 18-8.png|240px]]</div> | |||
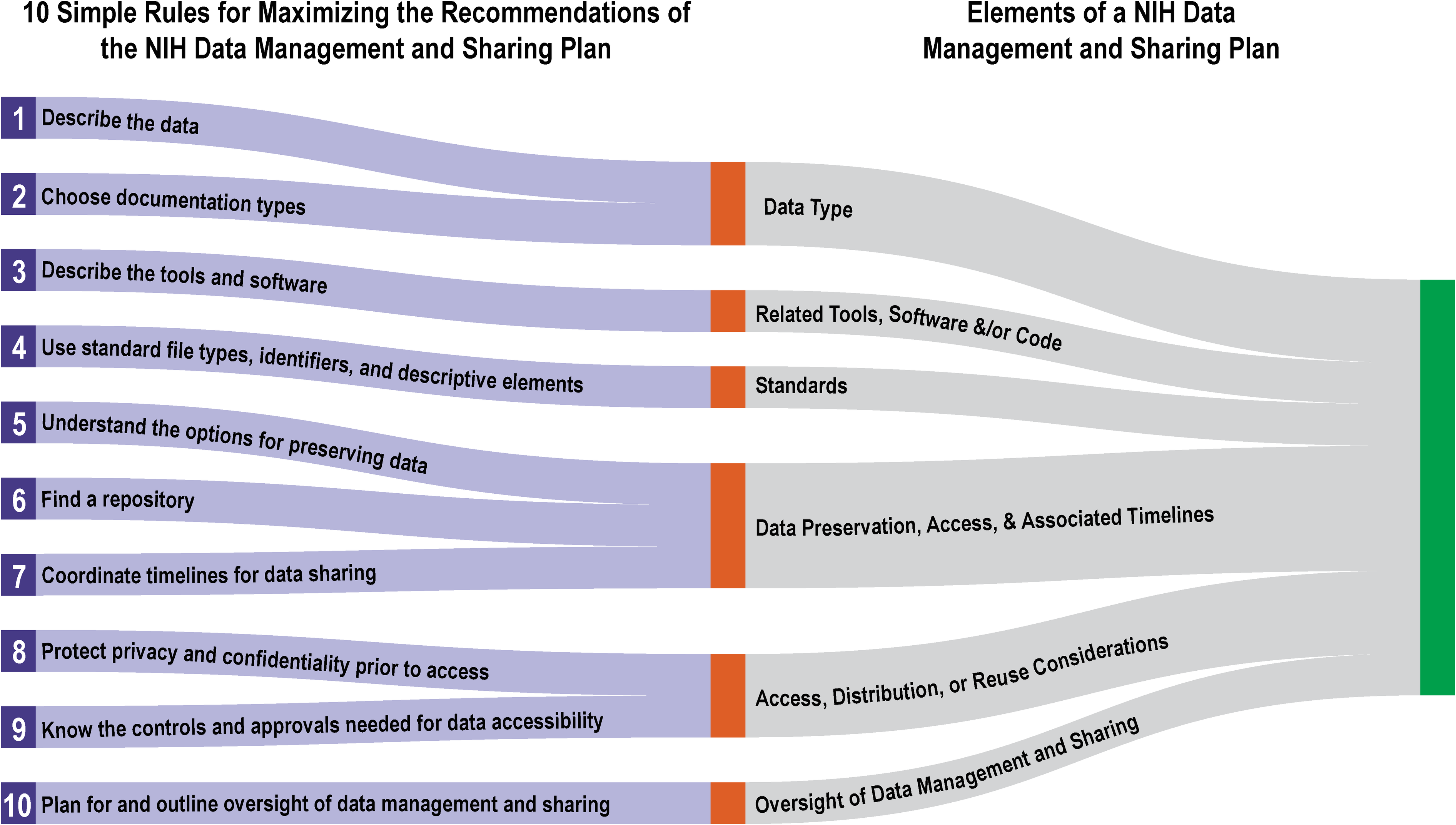
'''"[[Journal:Ten simple rules for maximizing the recommendations of the NIH data management and sharing plan|Ten simple rules for maximizing the recommendations of the NIH data management and sharing plan]]"''' | |||
The [[National Institutes of Health]] (NIH) Policy for Data Management and Sharing (DMS Policy) recognizes the NIH’s role as a key steward of the United States' biomedical research and information and seeks to enhance that stewardship through systematic recommendations for the preservation and [[Data sharing|sharing]] of research data generated by funded projects. The policy is effective as of January 2023. The recommendations include a requirement for the submission of a data management and sharing plan (DMSP) with funding applications, and while no strict template was provided, the NIH has released supplemental draft guidance on elements to consider when developing such a plan. This article provides 10 key recommendations for creating a DMSP that is both maximally compliant and effective. ('''[[Journal:Ten simple rules for maximizing the recommendations of the NIH data management and sharing planFull article...]]''')<br /> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: July 24–30:</h2> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig1 Taherdoost Electronics22 11-14.png|240px]]</div> | |||
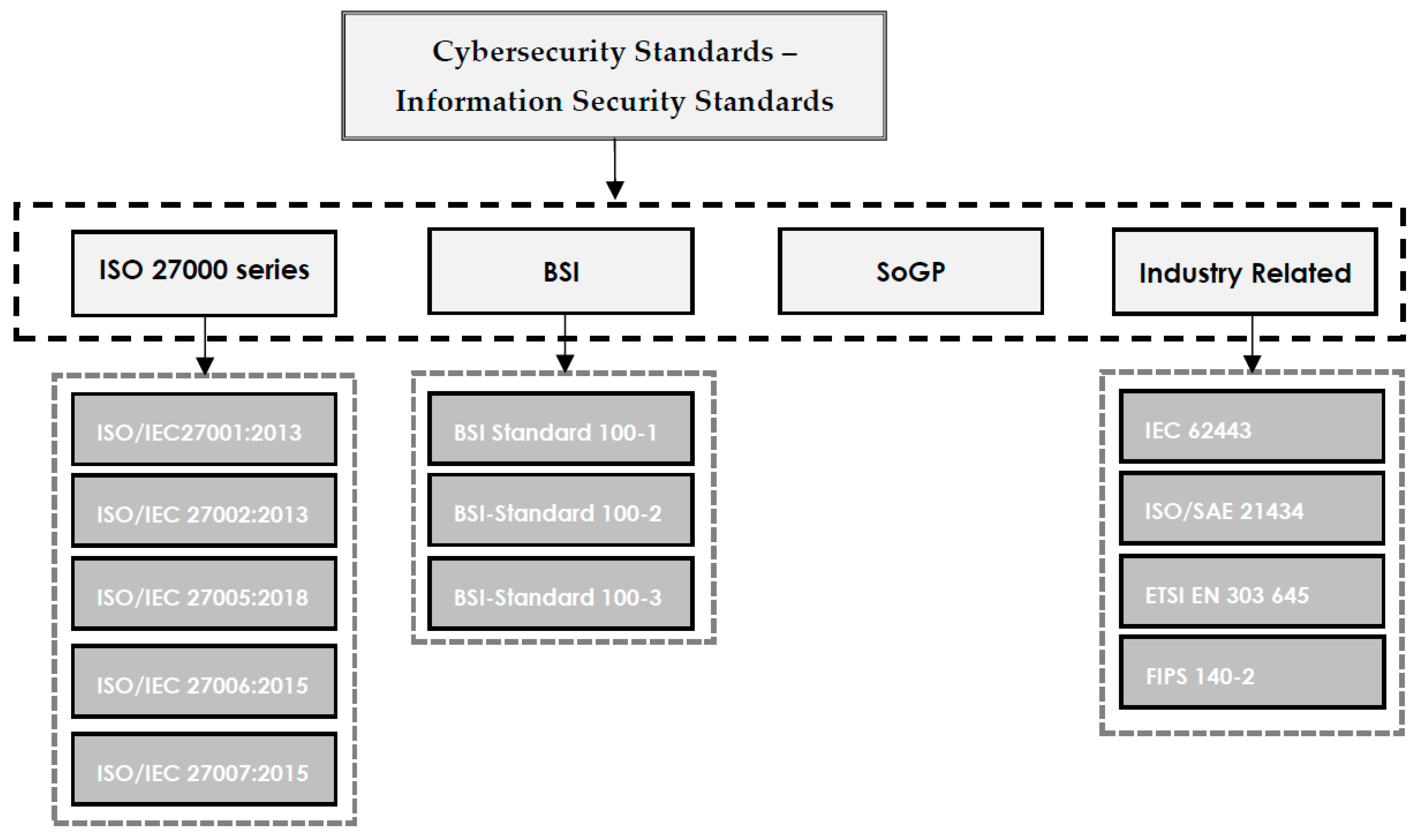
'''"[[Journal:Understanding cybersecurity frameworks and information security standards: A review and comprehensive overview|Understanding cybersecurity frameworks and information security standards: A review and comprehensive overview]]"''' | |||
Businesses are reliant on data to survive in the competitive market, and data is constantly in danger of loss or theft. Loss of valuable data leads to negative consequences for both individuals and organizations. [[Cybersecurity]] is the process of protecting sensitive data from damage or theft. To successfully achieve the objectives of implementing cybersecurity at different levels, a range of procedures and standards should be followed. Cybersecurity standards determine the requirements that an organization should follow to achieve cybersecurity objectives and minimize the impact of cybercrimes. Cybersecurity standards demonstrate whether an [[information management]] system can meet security requirements through a range of best practices and procedures. A range of standards has been established by various organizations to be employed in information management systems of different sizes and types ... ('''[[Journal:Understanding cybersecurity frameworks and information security standards: A review and comprehensive overview|Full article...]]''')<br /> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: July 17–23:</h2> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig4 Panse JofIntegBioinfo2022 19-4.jpg|240px]]</div> | |||
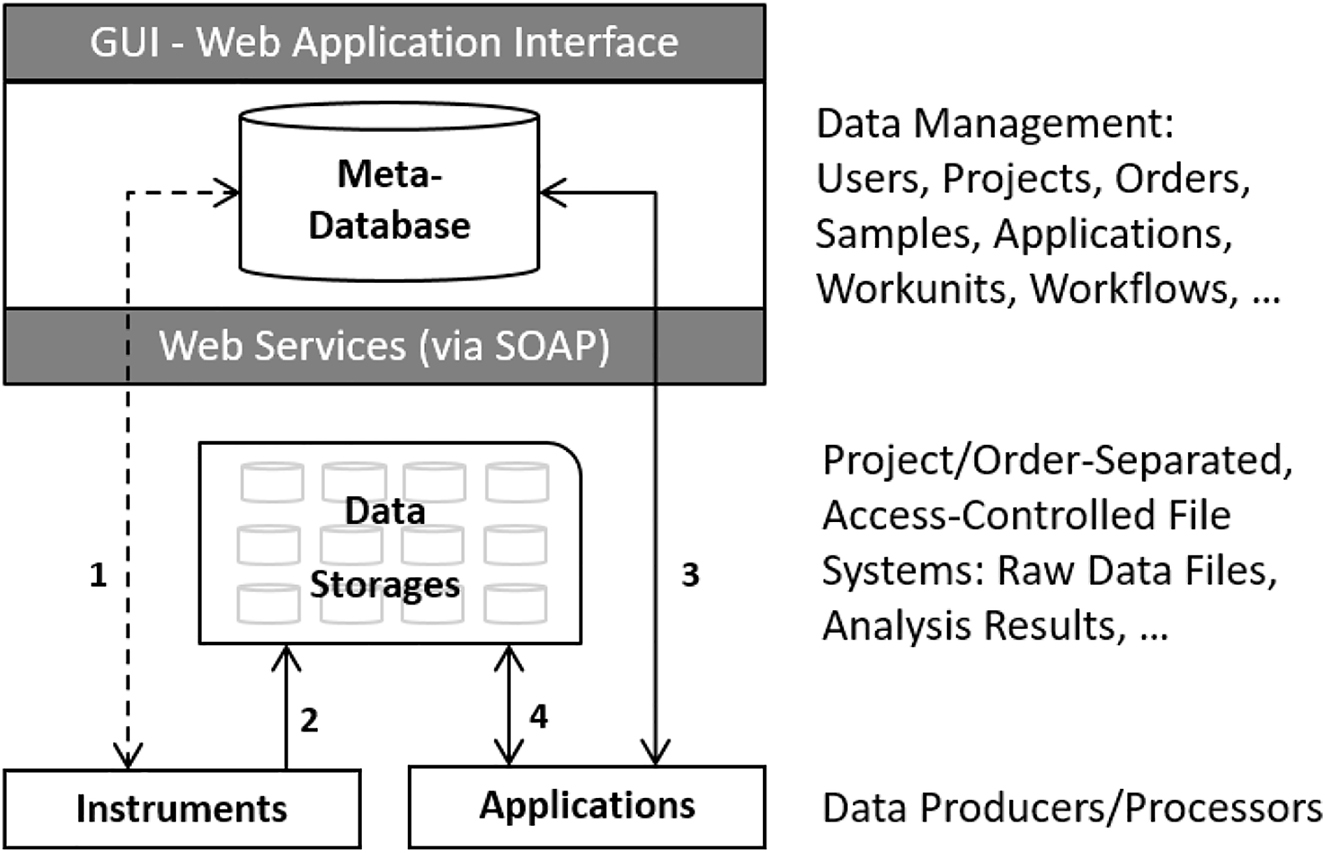
'''"[[Journal:Bridging data management platforms and visualization tools to enable ad-hoc and smart analytics in life sciences|Bridging data management platforms and visualization tools to enable ad-hoc and smart analytics in life sciences]]"''' | |||
Core facilities, which share centralized research resources across institutions and organizations, have to offer technologies that best serve the needs of their users and provide them a competitive advantage in research. They have to set up and maintain tens to hundreds of instruments, which produce large amounts of data and serve thousands of active projects and customers. Particular emphasis has to be given to the reproducibility of the results. Increasingly, the entire process—from building the research hypothesis, conducting the experiments, and taking the measurements, through to data exploration and [[Data analysis|analysis]]—is solely driven by very few experts in various scientific fields ... ('''[[Journal:Bridging data management platforms and visualization tools to enable ad-hoc and smart analytics in life sciences|Full article...]]''')<br /> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: July 10–16:</h2> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig4 Mustapää ApplSciences22 12-15.png|240px]]</div> | |||
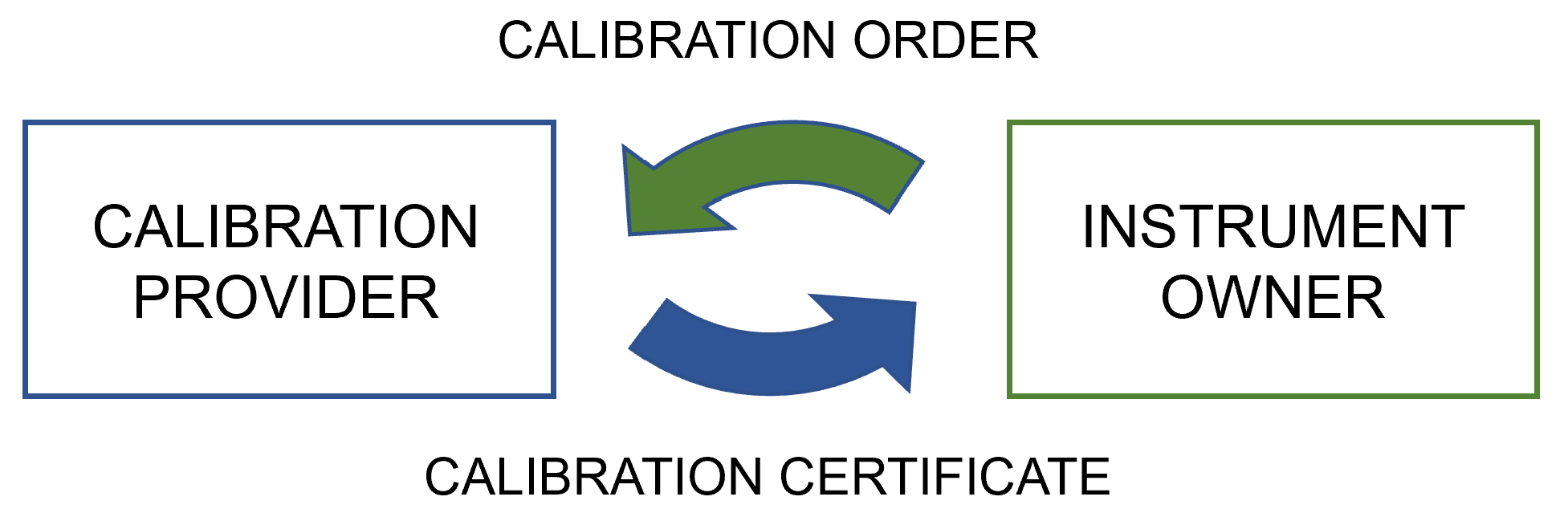
'''"[[Journal:Digitalization of calibration data management in the pharmaceutical industry using a multitenant platform|Digitalization of calibration data management in the pharmaceutical industry using a multitenant platform]]"''' | |||
The global [[Quality (business)|quality]] infrastructure (QI) has been established and is maintained to ensure the safety of products and services for their users. One of the cornerstones of the QI is [[metrology]], i.e., the science of measurement, as [[quality management system]]s (QMS) commonly rely on measurements for evaluating quality. For this reason, calibration procedures and management of the data related to them are of the utmost importance for quality management in the process industry, made a particularly high priority by [[Regulatory compliance|regulatory authorities]]. To overcome the relatively low level of digitalization in metrology, machine-interpretable data formats such as digital calibration certificates (DCC) are being developed ... ('''[[Journal:Digitalization of calibration data management in the pharmaceutical industry using a multitenant platform|Full article...]]''')<br /> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: July 03–09:</h2> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig2 Prahladh EgyptJofForSci22 12.png|240px]]</div> | |||
'''"[[Journal:Introductory evidence on data management and practice systems of forensic autopsies in sudden and unnatural deaths: A scoping review|Introductory evidence on data management and practice systems of forensic autopsies in sudden and unnatural deaths: A scoping review]]"''' | |||
The investigation into sudden unexpected and unnatural deaths supports criminal justice, aids in litigation, and provides important information for [[public health]], including surveillance, [[epidemiology]], and prevention programs. The use of mortality data to convey trends can inform policy development and resource allocations. Hence, data practices and [[Information management|data management systems]] in [[Forensic science|forensic medicine]] are critical. This study scoped literature and described the body of knowledge on data management and practice systems in forensic medicine. Five steps of the methodological framework of Arksey and O’Malley guided this scoping review. A combination of keywords, Boolean terms, and medical subject headings was used to search [[PubMed]], EBSCOhost (CINAHL with full text and Health Sources), Cochrane Library, Scopus, Web of Science, Science Direct, WorldCat, and Google Scholar for peer review papers in English ... ('''[[Journal:Introductory evidence on data management and practice systems of forensic autopsies in sudden and unnatural deaths: A scoping review|Full article...]]''')<br /> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: June 26–July 02:</h2> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig3 Snyder PLOSDigHlth22 1-11.png|240px]]</div> | |||
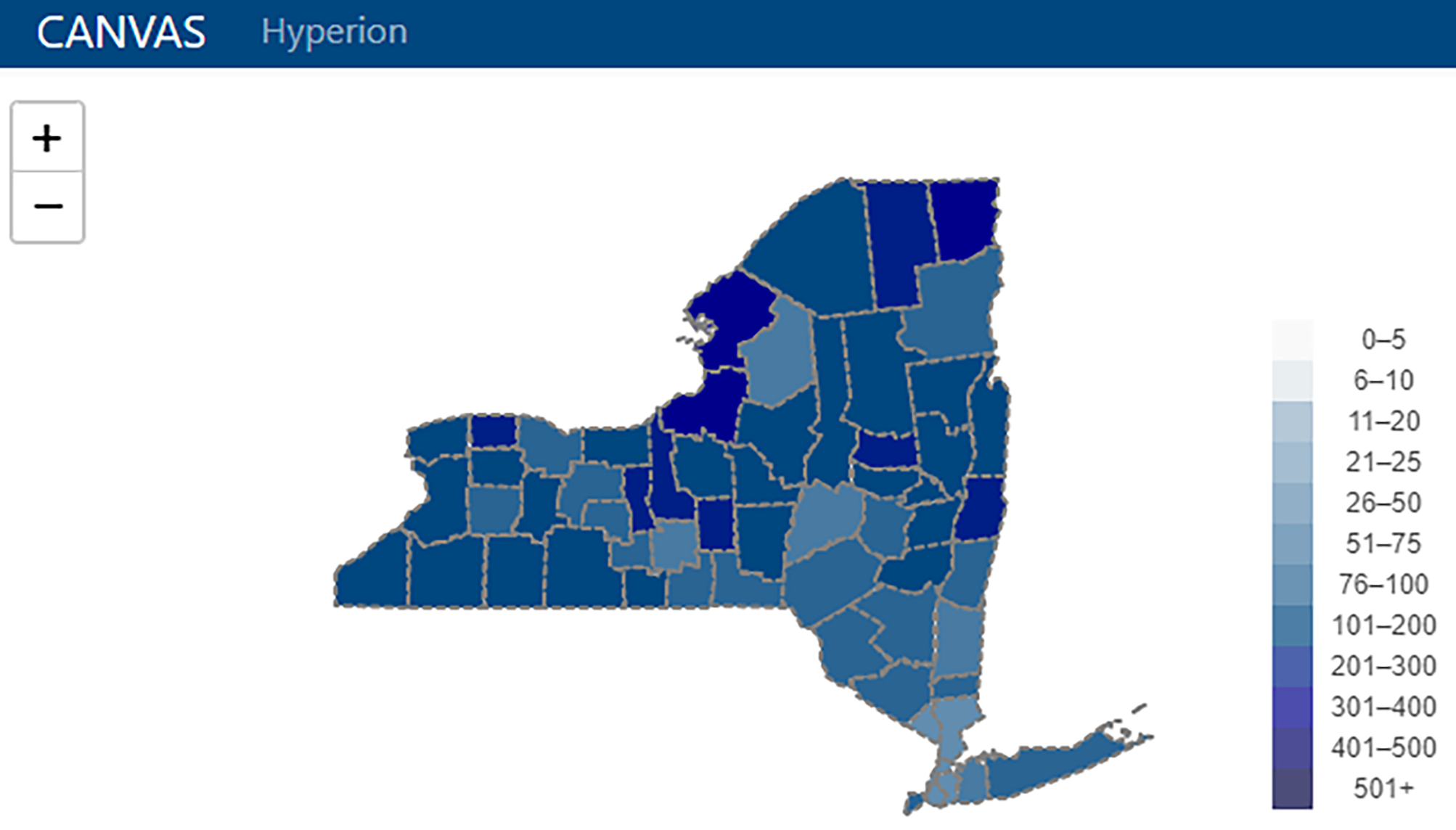
'''"[[Journal:From months to minutes: Creating Hyperion, a novel data management system expediting data insights for oncology research and patient care|From months to minutes: Creating Hyperion, a novel data management system expediting data insights for oncology research and patient care]]"''' | |||
Ensuring timely access to accurate data is critical for the functioning of a [[cancer]] center. Despite overlapping data needs, data are often fragmented and sequestered across multiple systems (such as the [[electronic health record]] [EHR], state and federal registries, and research [[database]]s), creating high barriers to data access for clinicians, researchers, administrators, quality officers, and patients. The creation of [[System integration|integrated data systems]] also faces technical, leadership, cost, and human resource barriers, among others. The University of Rochester's James P. Wilmot Cancer Institute (WCI) hired a small team of individuals with both technical and clinical expertise to develop a custom [[Information management|data management]] software platform—Hyperion— addressing five challenges: lowering the skill level required to maintain the system, reducing costs, allowing users to access data autonomously, optimizing [[Information security|data security]] and utilization, and shifting technological team structure to encourage rapid innovation ... ('''[[Journal:From months to minutes: Creating Hyperion, a novel data management system expediting data insights for oncology research and patient care|Full article...]]''')<br /> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: June 19–25:</h2> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig1 Scheibner BMCMedEthics22 23.png|240px]]</div> | |||
'''"[[Journal:Health data privacy through homomorphic encryption and distributed ledger computing: An ethical-legal qualitative expert assessment study|Health data privacy through homomorphic encryption and distributed ledger computing: An ethical-legal qualitative expert assessment study]]"''' | |||
Increasingly, [[hospital]]s and research institutes are developing technical solutions for [[Data sharing|sharing patient data]] in a [[Information privacy|privacy-preserving manner]]. Two of these technical solutions are [[homomorphic encryption]] and [[Blockchain|distributed ledger]] technology. Homomorphic encryption allows computations to be performed on data without this data ever being decrypted. Therefore, homomorphic encryption represents a potential solution for conducting feasibility studies on cohorts of sensitive patient data stored in distributed locations. Distributed ledger technology provides a permanent record on all transfers and processing of patient data, allowing data custodians to audit access. A significant portion of the current literature has examined how these technologies might comply with data protection and research ethics frameworks ... ('''[[Journal:Health data privacy through homomorphic encryption and distributed ledger computing: An ethical-legal qualitative expert assessment study|Full article...]]''')<br /> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: June 12–18:</h2> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig4 Fernandes AQUA22 71-3.png|240px]]</div> | |||
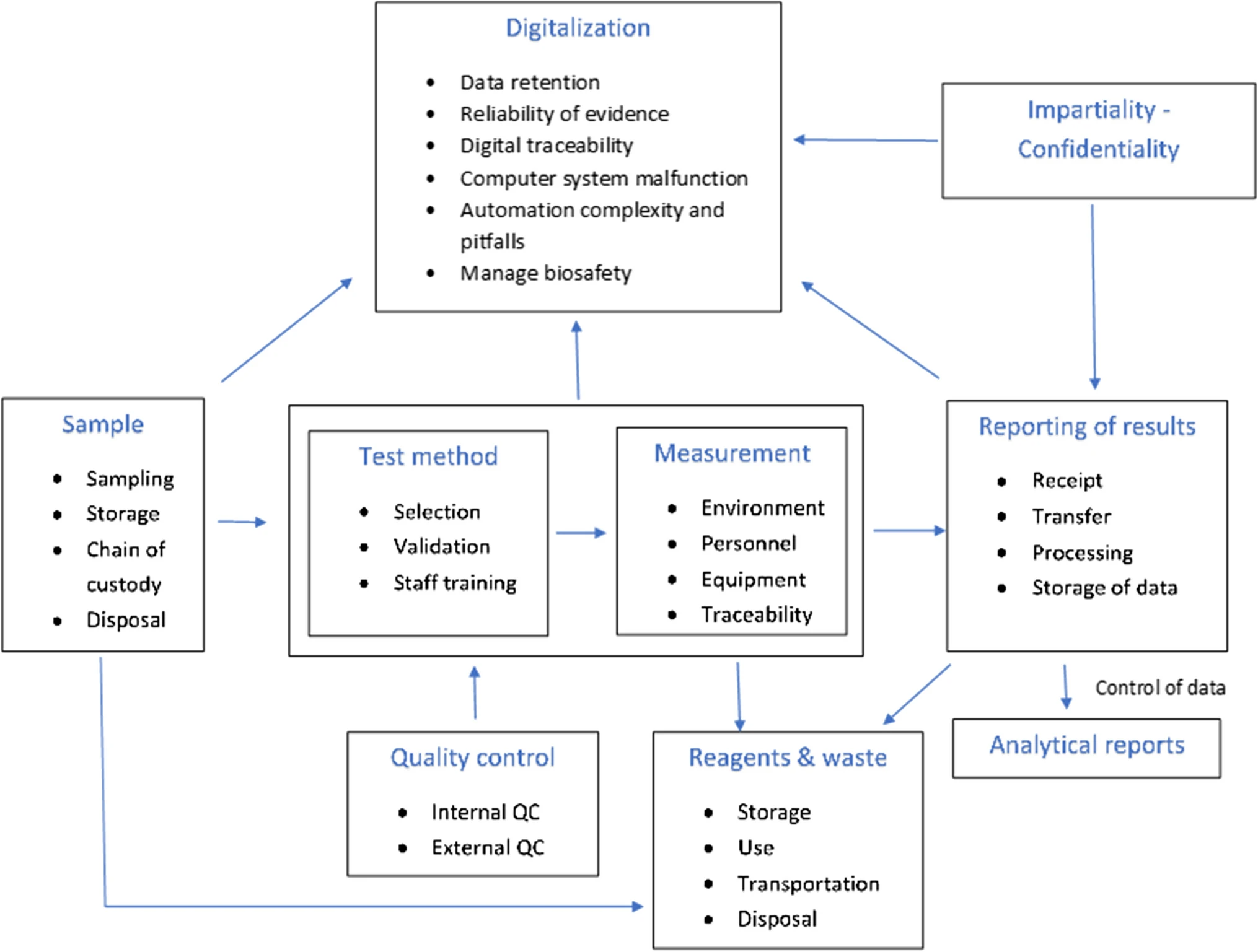
'''"[[Journal:Avoidance of operational sampling errors in drinking water analysis|Avoidance of operational sampling errors in drinking water analysis]]"''' | |||
The internal audits carried out in the first half of 2019 in Portuguese water [[Laboratory|laboratories]] as part of [[Quality (business)|quality]] accreditation in accordance with [[ISO/IEC 17025|ISO/IEC 17025:2017]] showed a high frequency of adverse events in connection with [[Sample (material)|sampling]]. These faults can be a consequence of a wide range of causes, and in some cases, the [[information]] about them can be insufficient or unclear. Considering that sampling has a major influence on the quality of the analytical results provided by water laboratories, this work presents a system for reporting and learning from adverse events. Its aim is to record nonconformities, errors, and adverse events, making possible automatic [[data analysis]] to better ensure [[Continual improvement process|continuous improvement]] in operational sampling. The system is based on the Eindhoven Classification Model and enables automatic data analysis and reporting to identify the main causes of failure ... ('''[[Journal:Avoidance of operational sampling errors in drinking water analysis|Full article...]]''')<br /> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: June 05–11:</h2> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig1 Miguel QuimicaNova22 44-6.png|240px]]</div> | |||
'''"[[Journal:ISO/IEC 17025: History and introduction of concepts|ISO/IEC 17025: History and introduction of concepts]]"''' | |||
[[Quality (business)|Quality]] is an increasingly present concept nowadays, and meeting the needs of customers who buy and use products and hire services becomes essential. For [[Laboratory|laboratories]], the concept is applied not only to the reliability and traceability of the results produced, but it also presents itself in meeting the customer’s needs and providing confidence when signing agreements in the international trade. The concept of quality in a laboratory can be carried out from the development and implementation of a [[quality management system]] (QMS). To this end, the normative, internationally accepted document [[ISO/IEC 17025]] aims at instructing the development and implementation of a management system, which ideally proves the technical capacity of testing and [[Reference laboratory|calibration laboratories]] and guides the generation of reliable results ... ('''[[Journal:ISO/IEC 17025: History and introduction of concepts|Full article...]]''')<br /> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: May 29–June 04:</h2> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig2 Pillai FrontBioengBiotech2022 10.jpg|120px]]</div> | |||
'''"[[Journal:Practical considerations for laboratories: Implementing a holistic quality management system|Practical considerations for laboratories: Implementing a holistic quality management system]]"''' | |||
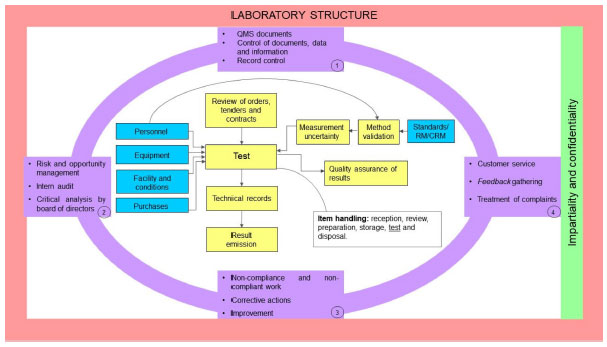
A [[quality management system]] (QMS) is an essential element for the effective operation of [[research]], clinical, testing, or production/manufacturing [[Laboratory|laboratories]]. As technology continues to rapidly advance and new challenges arise, laboratories worldwide have responded with innovation and process changes to meet the continued demand. It is critical for laboratories to maintain a robust QMS that accommodates laboratory activities (e.g., basic and applied research; regulatory, clinical, or proficiency testing), records management, and a path for [[Continual improvement process|continuous improvement]] to ensure that results and data are reliable, accurate, timely, and reproducible. A robust, suitable QMS provides a framework to address gaps and risks throughout the laboratory's [[workflow]] that could potentially lead to a critical error, thus compromising the integrity and credibility of the institution. While there are many QMS frameworks (e.g., a model such as a consensus standard, guideline, or regulation) that may apply to laboratories, ensuring that the appropriate framework is adopted based on the type of work performed and that key implementation steps are taken is important for the long-term success of the QMS and for the advancement of science ... ('''[[Journal:Practical considerations for laboratories: Implementing a holistic quality management system|Full article...]]''')<br /> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: May 22–28:</h2> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig2 Berciano FrontNutr2022 9.jpg|240px]]</div> | |||
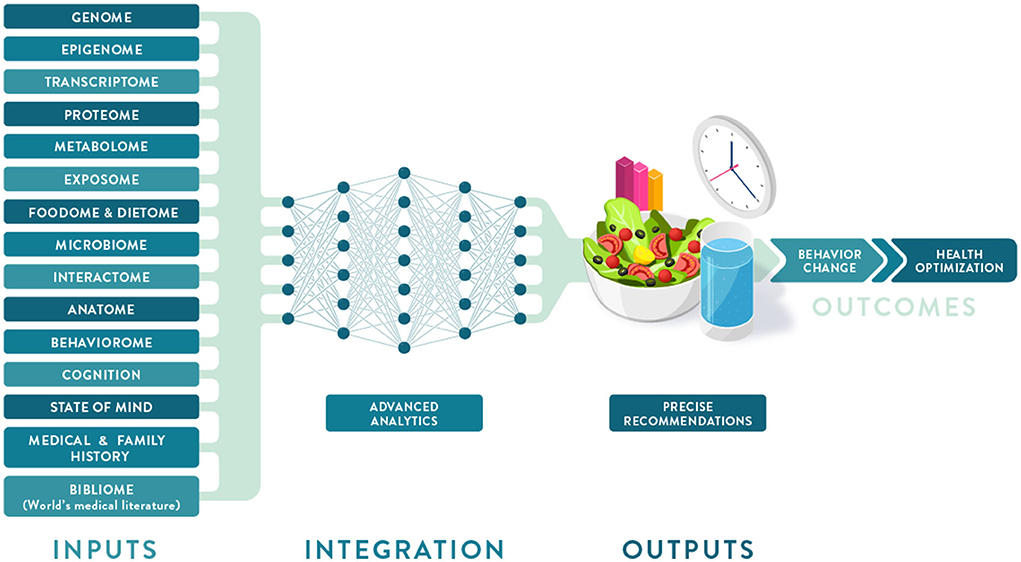
'''"[[Journal:Precision nutrition: Maintaining scientific integrity while realizing market potential|Precision nutrition: Maintaining scientific integrity while realizing market potential]]"''' | |||
Precision nutrition (PN) is an approach to developing comprehensive and dynamic [[Nutritional science|nutritional]] recommendations based on individual variables, including [[genetics]], [[microbiome]], [[Basic metabolic panel|metabolic profile]], health status, physical activity, dietary pattern, and food environment, as well as socioeconomic and psychosocial characteristics. PN can help answer the question “what should I eat to be healthy?”, recognizing that what is healthful for one individual may not be the same for another, and understanding that health and responses to diet change over time. The growth of the PN market has been driven by increasing consumer interest in individualized products and services coupled with advances in technology, analytics, and [[Omics|omic sciences]]. However, important concerns are evident regarding the adequacy of scientific substantiation supporting claims for current products and services. An additional limitation to accessing PN is the current cost of [[Medical test|diagnostic tests]] and wearable [[Medical device|devices]] ... ('''[[Journal:Precision nutrition: Maintaining scientific integrity while realizing market potential|Full article...]]''')<br /> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: May 15–21:</h2> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig1 daSilva Sustain22 14-22.png|240px]]</div> | |||
'''"[[Journal:Construction of control charts to help in the stability and reliability of results in an accredited water quality control laboratory|Construction of control charts to help in the stability and reliability of results in an accredited water quality control laboratory]]"''' | |||
Overall, [[laboratory]] water [[Quality (business)|quality]] analysis must have stability in their results, especially in laboratories accredited by [[ISO/IEC 17025]]. Accredited parameters should be strictly reliable. Using [[control chart]]s to ascertain divergences between results is thus very useful. The present work applied a methodology of [[Data analysis|analysis of results]] through control charts to accurately monitor the results for a wastewater treatment plant. The parameters analyzed were pH, biological oxygen demand for five days (BOD<sub>5</sub>), chemical oxygen demand (COD), total suspended solids (TSS), and total phosphorus (TP). The stability of the results was analyzed from the control charts and 30 analyses performed in the last 12 months. From the results, it was possible to observe whether the results were stable, according to the rehabilitation factor, which cannot exceed WN = 1.00, and the efficiency of removal of pollutants, which remained above 70% for all parameters ... ('''[[Journal:Construction of control charts to help in the stability and reliability of results in an accredited water quality control laboratory|Full article...]]''')<br /> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: May 08–14:</h2> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig1 Hong CancerInnov22 1-1.png|240px]]</div> | |||
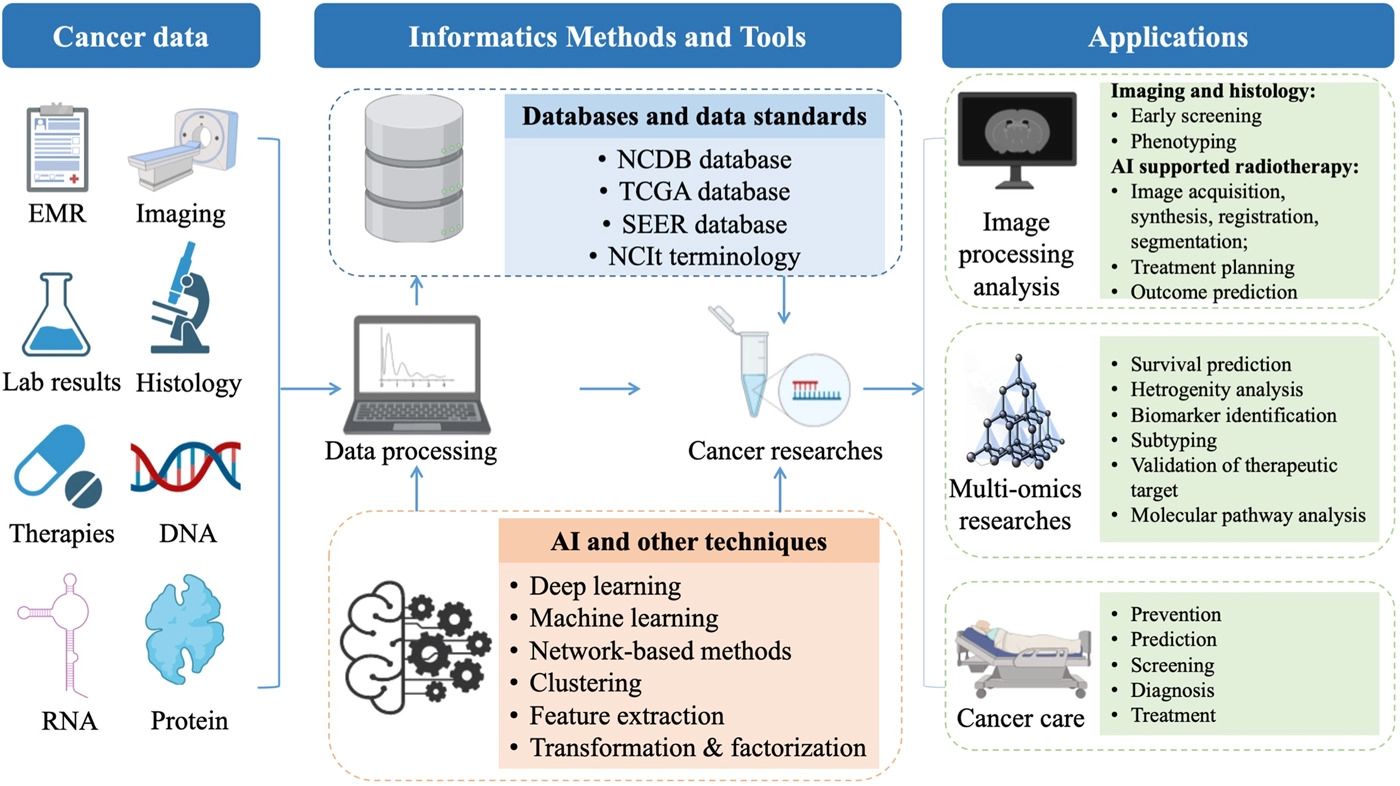
'''"[[Journal:Application of informatics in cancer research and clinical practice: Opportunities and challenges|Application of informatics in cancer research and clinical practice: Opportunities and challenges]]"''' | |||
[[Cancer informatics]] has significantly progressed in the big data era. We summarize the application of [[Informatics (academic field)|informatics]] approaches to the [[cancer]] domain from both the informatics perspective (e.g., [[Information management|data management]] and [[Information science|data science]]) and the clinical perspective (e.g., cancer screening, risk assessment, diagnosis, treatment, and prognosis). We discuss various informatics methods and tools that are widely applied in cancer research and practices, such as cancer databases, data standards, terminologies, high-throughput [[omics]] [[data mining]], [[machine learning]] algorithms, [[artificial intelligence]] [[imaging]], and intelligent radiation ... ('''[[Journal:Application of informatics in cancer research and clinical practice: Opportunities and challenges|Full article...]]''')<br /> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: May 01–07:</h2> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Box1 Szarfman CommMed22 2.png|240px]]</div> | |||
'''"[[Journal:Recommendations for achieving interoperable and shareable medical data in the USA|Recommendations for achieving interoperable and shareable medical data in the USA]]"''' | |||
Easy access to large quantities of accurate health data is required to understand medical and scientific [[information]] in real time; evaluate public health measures before, during, and after times of crisis; and prevent medical errors. Introducing a system in the United States of America that allows for efficient access to such health data and ensures auditability of data facts, while avoiding data silos, will require fundamental changes in current practices. Here, we recommend the implementation of standardized data collection and transmission systems, universal identifiers for individual patients and end users, a reference standard infrastructure to support calibration and integration of [[laboratory]] results from equivalent tests, and modernized working practices. Requiring comprehensive and binding [[Technical standard|standards]], rather than incentivizing voluntary and often piecemeal efforts for [[data exchange]], will allow us to achieve the analytical information environment that patients need ... ('''[[Journal:Recommendations for achieving interoperable and shareable medical data in the USA|Full article...]]''')<br /> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: April 24–30:</h2> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig2 Henrichs Sensors22 22-1.png|240px]]</div> | |||
'''"[[Journal:Can a byte improve our bite? An analysis of digital twins in the food industry|Can a byte improve our bite? An analysis of digital twins in the food industry]]"''' | |||
The food industry faces many challenges, including the need to feed a growing population, manage food loss and waste, and improve inefficient production systems. To cope with those challenges, [[digital twin]]s—digital representations of physical entities created by integrating real-time and real-world data—seem to be a promising approach. This paper aims to provide an overview of digital twin applications in the food industry and analyze their challenges and potentials. First, a literature review is executed to examine digital twin applications in the food supply chain. The applications found are classified according to a taxonomy, and key elements to implement digital twins are identified. Further, the challenges and potentials of digital twin applications in the food industry are discussed. This survey reveals that application of digital twins mainly target the production (i.e., agriculture) or food processing stages ... ('''[[Journal:Can a byte improve our bite? An analysis of digital twins in the food industry|Full article...]]''')<br /> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: April 17–23:</h2> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig1 Hatakeyama-Sato njpCompMat22 8.png|240px]]</div> | |||
'''"[[Journal:Exploration of organic superionic glassy conductors by process and materials informatics with lossless graph database|Exploration of organic superionic glassy conductors by process and materials informatics with lossless graph database]]"''' | |||
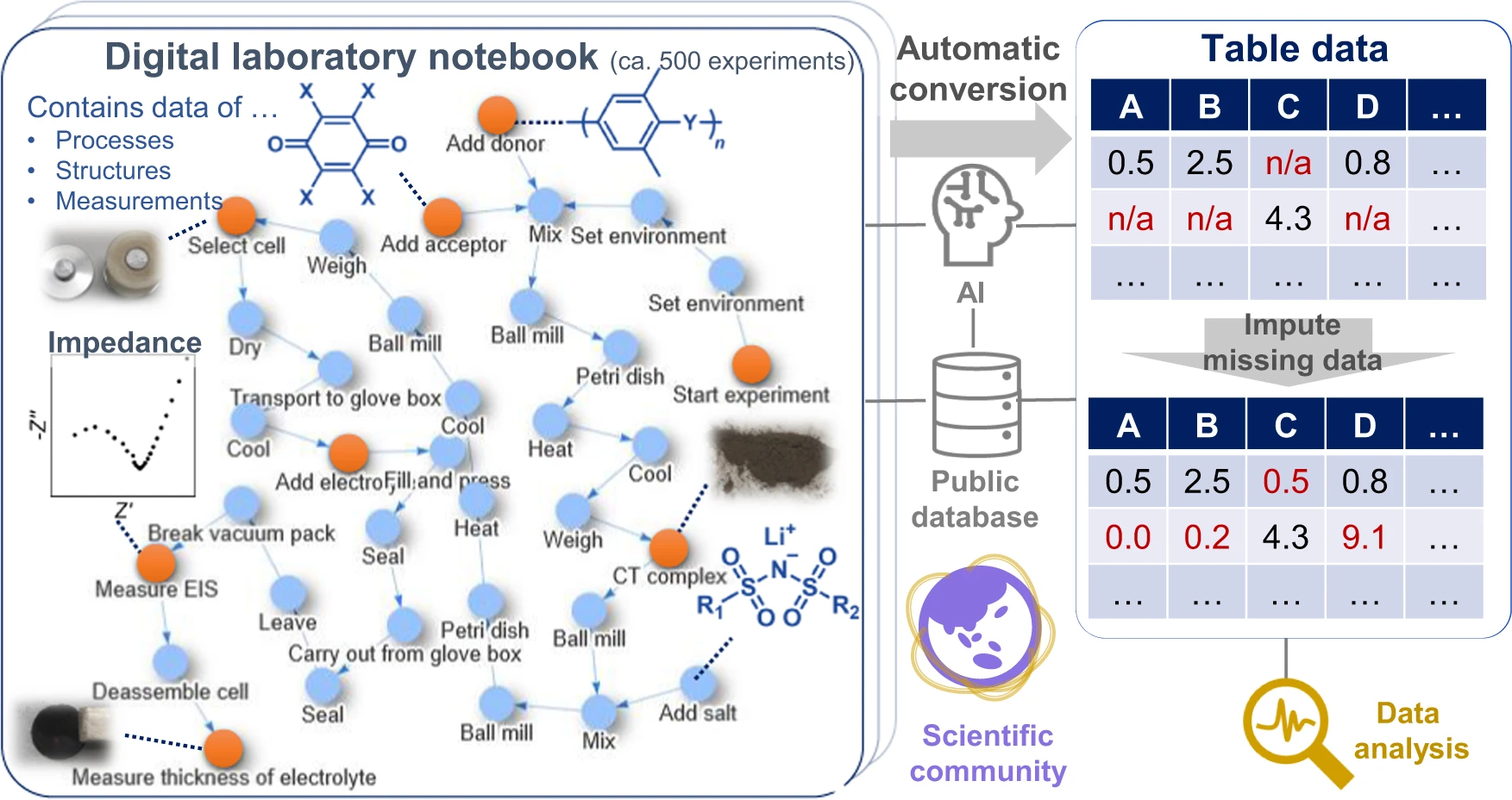
Data-driven [[Materials science|material exploration]] is a ground-breaking research style; however, daily experimental results are difficult to record, [[Data analysis|analyze]], and [[Data sharing|share]]. We report a data platform that losslessly describes the relationships of structures, properties, and processes as graphs in [[electronic laboratory notebook]]s (ELNs). As a model project, organic [[wikipedia:Fast ion conductor#Superionic conductors|superionic glassy conductors]] were explored by recording over 500 different experiments. Automated data analysis revealed the essential factors for a remarkable room-temperature ionic conductivity of 10<sup>−4</sup> to 10<sup>−3</sup> S cm<sup>−1</sup> and a Li<sup>+</sup> transference number of around 0.8. In contrast to previous materials research, everyone can access all the experimental results—including graphs, raw measurement data, and data processing systems—at a public repository. Direct data sharing will improve scientific communication and accelerate integration of material knowledge ... ('''[[Journal:Exploration of organic superionic glassy conductors by process and materials informatics with lossless graph database|Full article...]]''')<br /> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: April 10–16:</h2> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig1 Huang iScience2022 25-8.jpg|240px]]</div> | |||
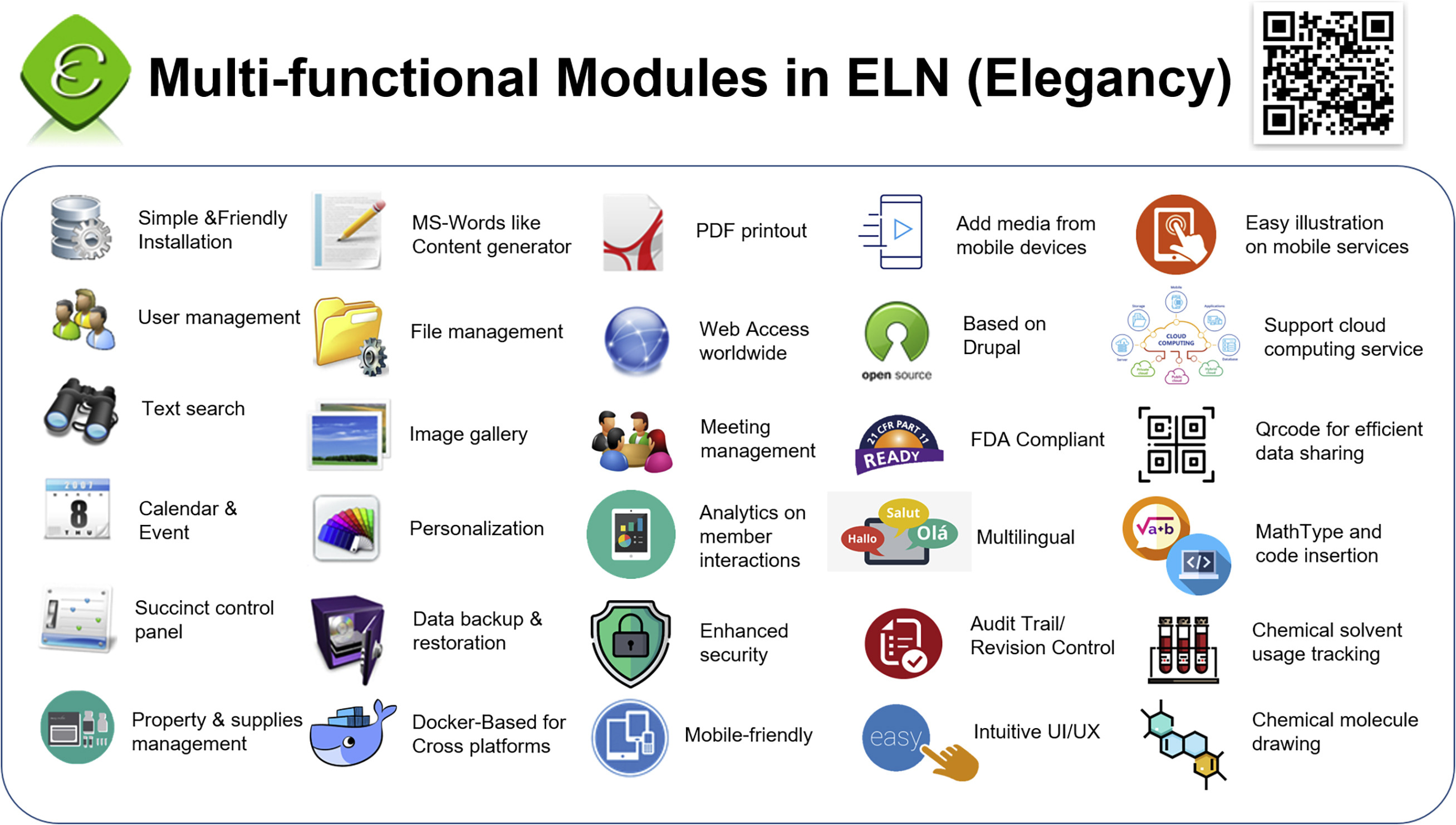
'''"[[Journal:Elegancy: Digitizing the wisdom from laboratories to the cloud with free no-code platform|Elegancy: Digitizing the wisdom from laboratories to the cloud with free no-code platform]]"''' | |||
One of the top priorities in any [[laboratory]] is [[Archival informatics|archiving]] experimental data in the most secure, efficient, and errorless way. It is especially important to those in chemical and biological research, for it is more likely to damage experiment records. In addition, the transmission of experiment results from paper to electronic devices is time-consuming and redundant. Therefore, we introduce an [[Open-source software|open-source]] no-code [[electronic laboratory notebook]] (ELN), Elegancy, a [[Cloud computing|cloud-based]]/standalone web service distributed as a Docker image. Elegancy fits all laboratories but is specially equipped with several features benefitting biochemical laboratories. It can be accessed via various web browsers, allowing researchers to upload photos or audio recordings directly from their mobile devices. Elegancy also contains a meeting arrangement module, audit/revision control, and laboratory supply management system. We believe Elegancy could help the scientific research community gather evidence, share information, reorganize knowledge, and digitize laboratory works with greater ease and security ... ('''[[Journal:Elegancy: Digitizing the wisdom from laboratories to the cloud with free no-code platform|Full article...]]''')<br /> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: April 03–09:</h2> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig2 Foster JoMedLibAss22 110-2.png|240px]]</div> | |||
'''"[[Journal:Implementing an institution-wide electronic laboratory notebook initiative|Implementing an institution-wide electronic laboratory notebook initiative]]"''' | |||
To strengthen institutional research [[Information management|data management]] practices, the [[Indiana University – Purdue University Indianapolis|Indiana University School of Medicine]] (IUSM) licensed an [[electronic laboratory notebook]] (ELN) to improve the organization, security, and shareability of [[information]] and data generated by the school’s researchers. The Ruth Lilly Medical Library led implementation on behalf of the IUSM’s Office of Research Affairs. This article describes the pilot and full-scale implementation of an ELN at IUSM. The initial pilot of the ELN in late 2018 involved 15 research labs, with access expanded in 2019 to all academic medical school constituents ... ('''[[Journal:Implementing an institution-wide electronic laboratory notebook initiative|Full article...]]''')<br /> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: March 27–April 02:</h2> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig3 Ronalter EnviroDevSust22 660.png|240px]]</div> | |||
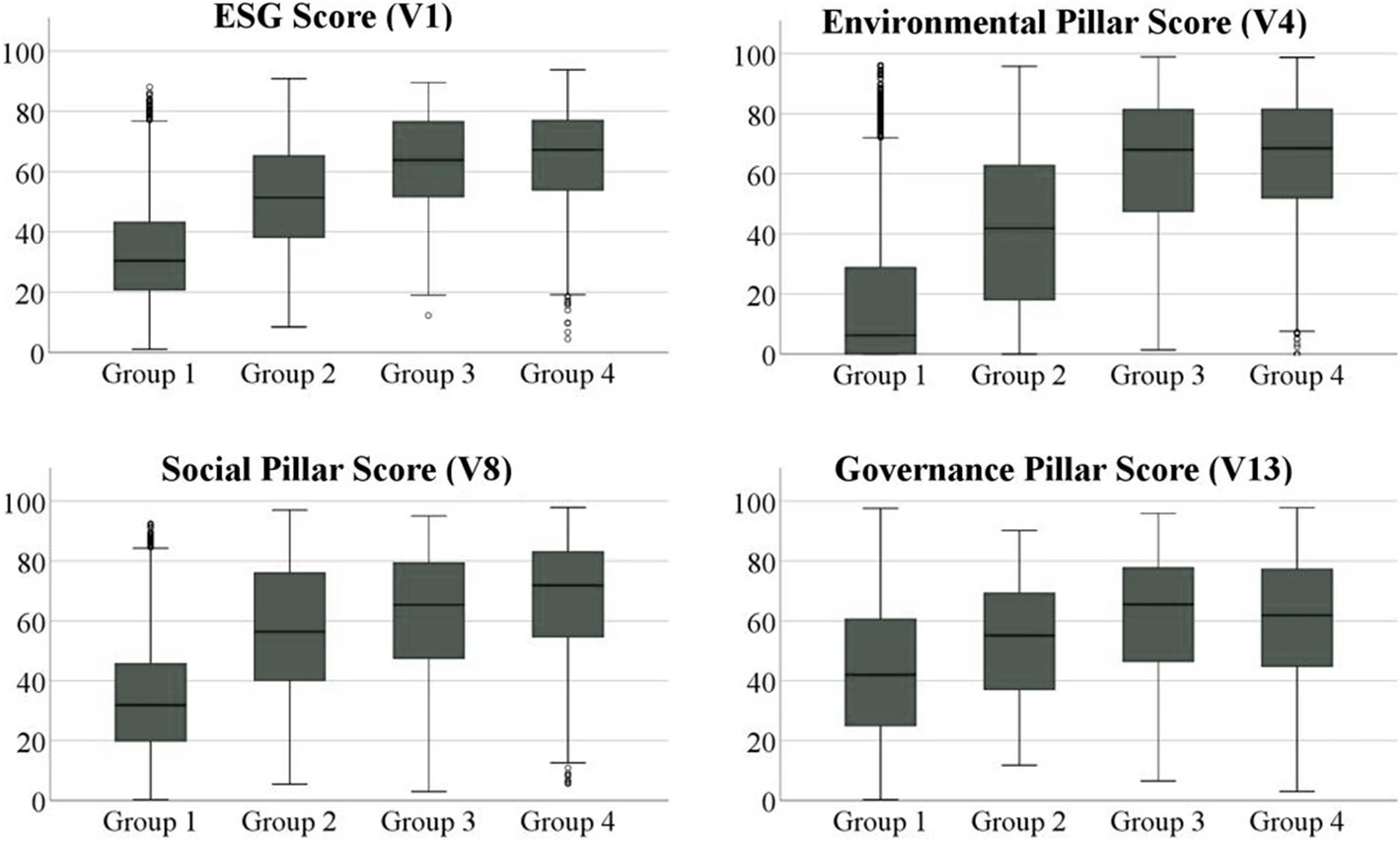
'''"[[Journal:Quality and environmental management systems as business tools to enhance ESG performance: A cross-regional empirical study|Quality and environmental management systems as business tools to enhance ESG performance: A cross-regional empirical study]]"''' | |||
The growing societal and political focus on sustainability at the global level is pressuring companies to enhance their [[wikipedia:Environmental, social, and corporate governance|environmental, social, and governance]] (ESG) performance to satisfy respective stakeholder needs and ensure sustained business success. With a data sample of 4,292 companies from Europe, East Asia, and North America, this work aims to prove through a cross-regional empirical study that [[quality management system]]s (QMSs) and [[environmental management system]]s (EMSs) represent powerful business tools to achieve this enhanced ESG performance. Descriptive and cluster analyses reveal that firms with QMSs and/or EMSs accomplish statistically significant higher ESG scores than companies without such management systems. Furthermore, the results indicate that operating both types of management systems simultaneously increases performance in the environmental and social pillar even further, while the governance dimension appears to be affected mainly by the adoption of EMSs alone ... ('''[[Journal:Quality and environmental management systems as business tools to enhance ESG performance: A cross-regional empirical study|Full article...]]''')<br /> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: March 20–26:</h2> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:GA Terlouw JofCheminfo22 14.png|240px]]</div> | |||
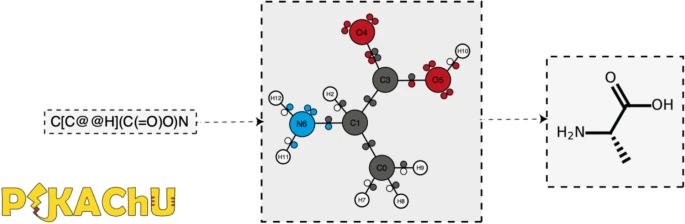
'''"[[Journal:PIKAChU: A Python-based informatics kit for analyzing chemical units|PIKAChU: A Python-based informatics kit for analyzing chemical units]]"''' | |||
As efforts to computationally describe and simulate the biochemical world become more commonplace, computer programs that are capable of ''in silico'' chemistry play an increasingly important role in biochemical research. While such programs exist, they are often dependency-heavy, difficult to navigate, or not written in [[Python (programming language)|Python]], the programming language of choice for [[Bioinformatics|bioinformaticians]]. Here, we introduce PIKAChU (Python-based Informatics Kit for Analysing CHemical Units), a [[Chemical informatics|cheminformatics]] toolbox with few dependencies implemented in Python. PIKAChU builds comprehensive molecular graphs from simplified molecular-input line-entry system (SMILES) strings, which allow for easy downstream analysis and visualization of molecules. ... ('''[[Journal:PIKAChU: A Python-based informatics kit for analyzing chemical units|Full article...]]''')<br /> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: March 13–19:</h2> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:GA Karim JofKSUScience2022 34-2.jpg|240px]]</div> | |||
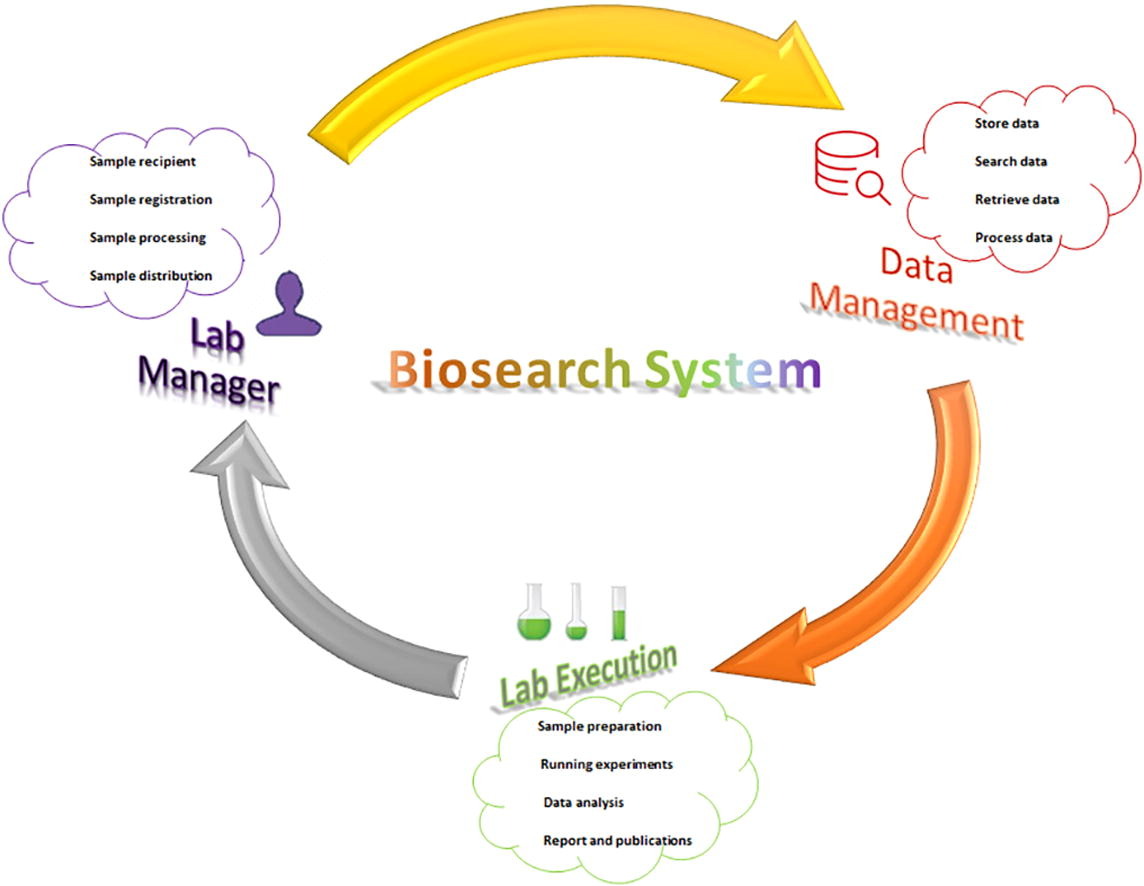
'''"[[Journal:Development of Biosearch System for biobank management and storage of disease-associated genetic information|Development of Biosearch System for biobank management and storage of disease-associated genetic information]]"''' | |||
Databases and software are important to manage modern high-throughput [[Laboratory|laboratories]] and store clinical and [[Genome informatics|genomic information]] for [[quality assurance]]. Commercial software is expensive, with proprietary code issues, while academic versions have adaptation issues. Our aim was to develop an adaptable in-house software system that can store specimen- and disease-associated genetic information in [[biobank]]s to facilitate [[translational research]]. A prototype was designed per the research requirements, and computational tools were used to develop the software under three tiers, using Visual Basic and ASP.net for the presentation tier, SQL Server for the data tier, and Ajax and JavaScript for the business tier. We retrieved specimens from the biobank using this software and performed microarray-based transcriptomic analysis to detect differentially expressed genes (DEGs) ... ('''[[Journal:Development of Biosearch System for biobank management and storage of disease-associated genetic information|Full article...]]''')<br /> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: March 06–12:</h2> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig1 Andrés-Hernández FrontNut2022 9.jpg|240px]]</div> | |||
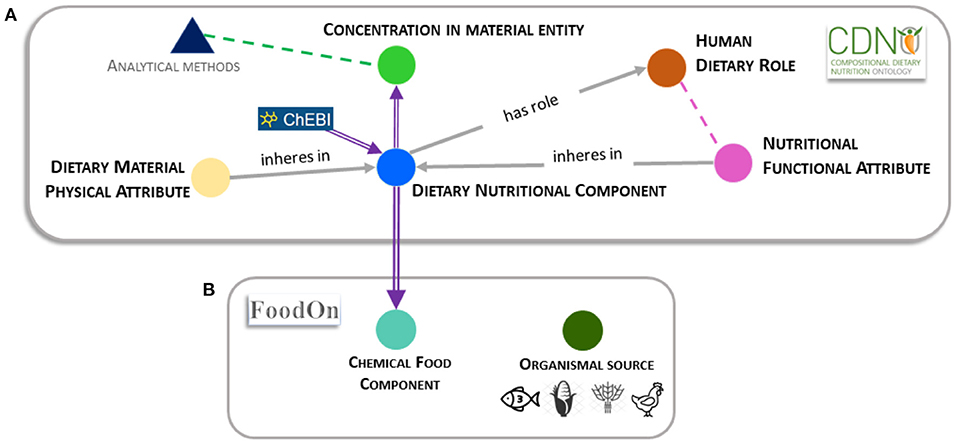
'''"[[Journal:Establishing a common nutritional vocabulary: From food production to diet|Establishing a common nutritional vocabulary: From food production to diet]]"''' | |||
Informed policy and decision-making for food systems, [[Food security|nutritional security]], and global health would benefit from standardization and comparison of food composition data, spanning production to consumption. To address this challenge, we present a formal [[controlled vocabulary]] of terms, definitions, and relationships within the [https://www.cdno.info/ Compositional Dietary Nutrition Ontology] (CDNO) that enables description of nutritional attributes for material entities contributing to the human diet. We demonstrate how ongoing community development of CDNO classes can harmonize trans-disciplinary approaches for describing [[Nutritional science|nutritional components]] from food production to diet ... ('''[[Journal:Establishing a common nutritional vocabulary: From food production to diet|Full article...]]''')<br /> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: February 28–March 05:</h2> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig2 Melanitis MATECWebConf21 349.png|240px]]</div> | |||
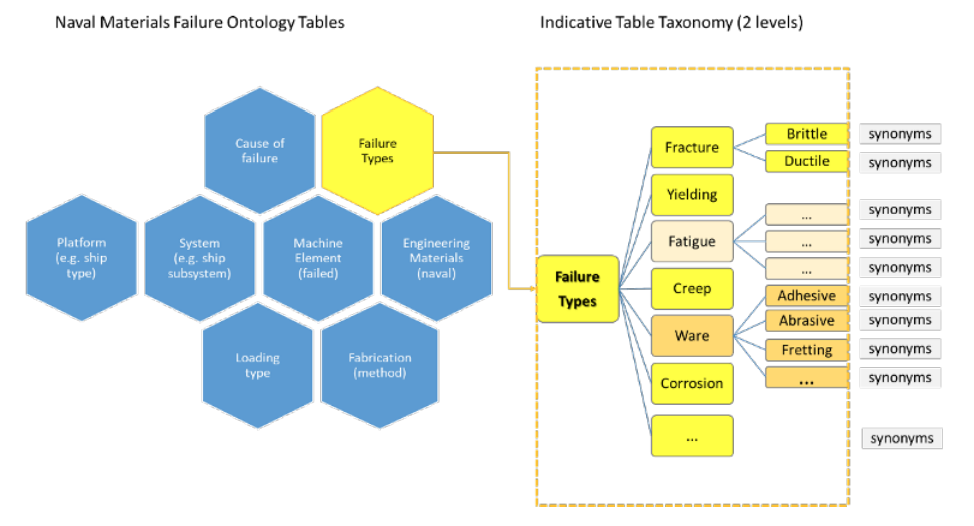
'''"[[Journal:Designing a knowledge management system for naval materials failures|Designing a knowledge management system for naval materials failures]]"''' | |||
Implemented materials fail from time to time, requiring [[failure analysis]]. This type of scientific analysis expands into [[Forensic science|forensic]] engineering for it aims not only to identify individual and symptomatic reasons for failure, but also to assess and understand repetitive failure patterns, which could be related to underlying material faults, design mistakes, or maintenance omissions. Significant [[information]] can be gained and studied from carefully documenting and managing the data that comes from failure analysis of materials, including in the naval industry. The NAVMAT research project, presented herein, attempts an interdisciplinary approach to [[materials informatics]] by integrating materials engineering and [[Informatics (academic field)|informatics]] under a platform of [[Information management|knowledge management]]. Our approach utilizes a focused, common-cause failure analysis methodology for the naval and marine environment. The platform's design is dedicated to the effective recording, efficient indexing, and easy and accurate retrieval of relevant information, including the associated history of maintenance and secure operation concerning failure incidents of marine materials, components, and systems in an organizational fleet. ... ('''[[Journal:Designing a knowledge management system for naval materials failures|Full article...]]''')<br /> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: February 20–27:</h2> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig2 Yoon LabAniRes22 38.png|240px]]</div> | |||
'''"[[Journal:Laboratory information management system for COVID-19 non-clinical efficacy trial data|Laboratory information management system for COVID-19 non-clinical efficacy trial data]]"''' | |||
As the number of large-scale research studies involving multiple organizations producing data has steadily increased, an integrated system for a common interoperable data format is needed. For example, in response to the [[coronavirus disease 2019]] (COVID-19) [[pandemic]], a number of global efforts are underway to develop vaccines and therapeutics. We are therefore observing an explosion in the proliferation of COVID-19 data, and interoperability is highly requested in multiple institutions participating simultaneously in COVID-19 pandemic research. In this study, a [[laboratory information management system]] (LIMS) has been adopted to systemically manage, via web interface, various COVID-19 non-clinical trial data—including mortality, clinical signs, body weight, body temperature, organ weights, viral titer (viral replication and viral RNA), and multi-organ [[histopathology]]—from multiple institutions ... ('''[[Journal:Laboratory information management system for COVID-19 non-clinical efficacy trial data|Full article...]]''')<br /> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: February 13–19:</h2> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig1 AbuHalimeh FrontBigData2022 5.jpg|240px]]</div> | |||
'''"[[Journal:Improving data quality in clinical research informatics tools|Improving data quality in clinical research informatics tools]]"''' | |||
Maintaining [[data quality]] is a fundamental requirement for any successful and long-term [[Information management|data management]] project. Providing high-quality, reliable, and statistically sound data is a primary goal for [[wikipedia:Clinical research|clinical research]] [[Informatics (academic field)|informatics]]. In addition, effective data governance and management are essential to ensuring accurate data counts, reports, and validation. As a crucial step of the clinical research process, it is important to establish and maintain organization-wide standards for data quality management to ensure consistency across all systems designed primarily for cohort identification ... ('''[[Journal:Improving data quality in clinical research informatics tools|Full article...]]''')<br /> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: February 6–12:</h2> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig1 Bohn JofLabMed2021 45-6.jpg|240px]]</div> | |||
'''"[[Journal:Electronic tools in clinical laboratory diagnostics: Key examples, limitations, and value in laboratory medicine|Electronic tools in clinical laboratory diagnostics: Key examples, limitations, and value in laboratory medicine]]"''' | |||
Electronic tools in [[clinical laboratory]] diagnostics can assist [[laboratory]] professionals, clinicians, and patients in medical diagnostic management and laboratory test interpretation. With increasing implementation of [[electronic health record]]s (EHRs) and [[laboratory information system]]s (LIS) worldwide, there is increasing demand for well-designed and evidence-based electronic resources. Both complex data-driven and simple interpretative electronic healthcare tools are currently available to improve the integration of clinical and laboratory [[information]] towards a more patient-centered approach to medicine. Several studies have reported positive clinical impact of electronic healthcare tool implementation in clinical laboratory diagnostics, including in the management of neonatal bilirubinemia, cardiac disease, and nutritional status ... ('''[[Journal:Electronic tools in clinical laboratory diagnostics: Key examples, limitations, and value in laboratory medicine|Full article...]]''')<br /> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: January 30–February 5:</h2> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig3 Dundar JofPathInfo2022 13.jpg|240px]]</div> | |||
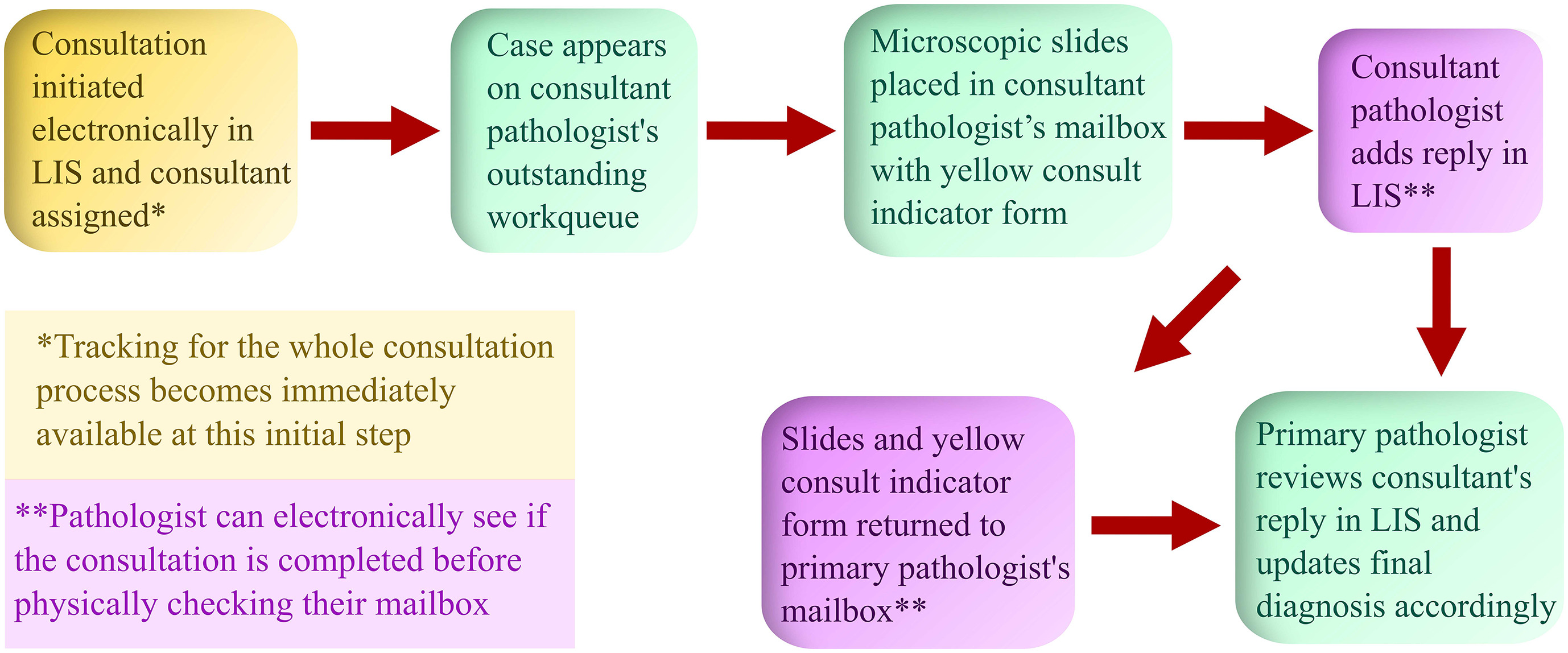
'''"[[Journal:Anatomic pathology quality assurance: Developing an LIS-based tracking and documentation module for intradepartmental consultations|Anatomic pathology quality assurance: Developing an LIS-based tracking and documentation module for intradepartmental consultations]]"''' | |||
An electronic intradepartmental consultation system for [[Anatomical pathology|anatomic pathology]] (AP) was conceived and developed in the [[laboratory information system]] (LIS) of University of Iowa Hospitals and Clinics in 2019. Previously, all surgical pathology intradepartmental consultative activities were initiated and documented with paper forms, which were circulated with the pertinent microscopic slides and were eventually filed. In this study, we discuss the implementation and utilization of an electronic intradepartmental AP consultation system. [[Workflow]]s and procedures were developed to organize intradepartmental surgical pathology consultations from the beginning to the end point of the consultative activities entirely using a paperless system that resided in the LIS ... ('''[[Journal:Anatomic pathology quality assurance: Developing an LIS-based tracking and documentation module for intradepartmental consultations|Full article...]]''')<br /> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: January 23–29:</h2> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig1 Sachdeva Information22 13-2.png|240px]]</div> | |||
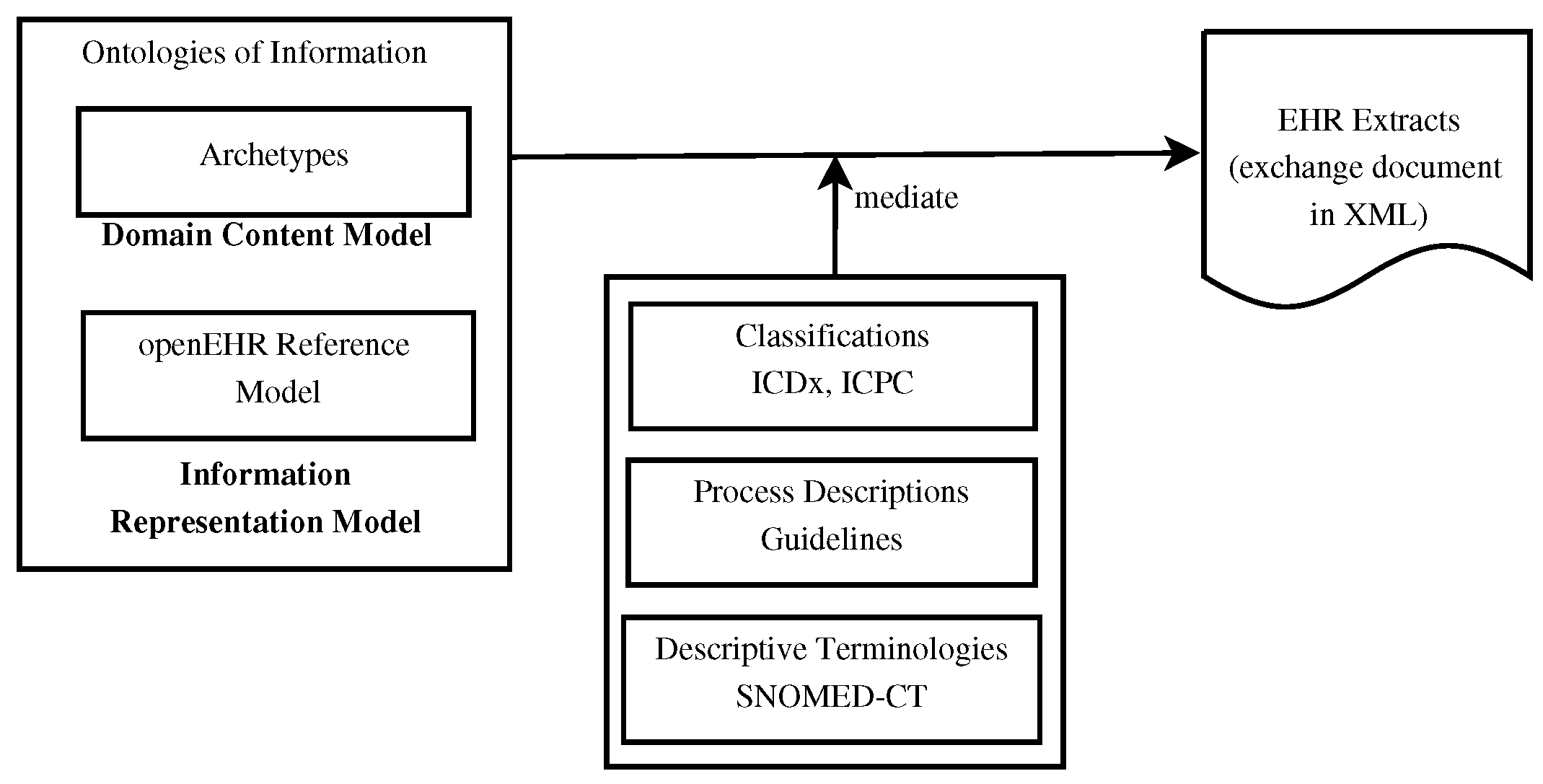
'''"[[Journal:Using knowledge graph structures for semantic interoperability in electronic health records data exchanges|Using knowledge graph structures for semantic interoperability in electronic health records data exchanges]]"''' | |||
[[Data sharing|Information sharing]] across medical institutions is restricted to [[information]] exchange between specific partners. The lifelong [[electronic health record]] (EHR) structure and content require standardization efforts. Existing standards such as [[openEHR]], [[Health Level 7]] (HL7), and ISO/EN 13606 aim to achieve data independence along with semantic interoperability. This study aims to discover knowledge representation to achieve semantic health data exchange. openEHR and ISO/EN 13606 use archetype-based technology for semantic interoperability. The HL7 Clinical Document Architecture is on its way to adopting this through HL7 templates. Archetypes are the basis for knowledge-based systems, as these are means to define clinical knowledge. The paper examines a set of formalisms for the suitability of describing, representing, and reasoning about archetypes ... ('''[[Journal:Using knowledge graph structures for semantic interoperability in electronic health records data exchanges|Full article...]]''')<br /> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: January 16–22:</h2> | |||
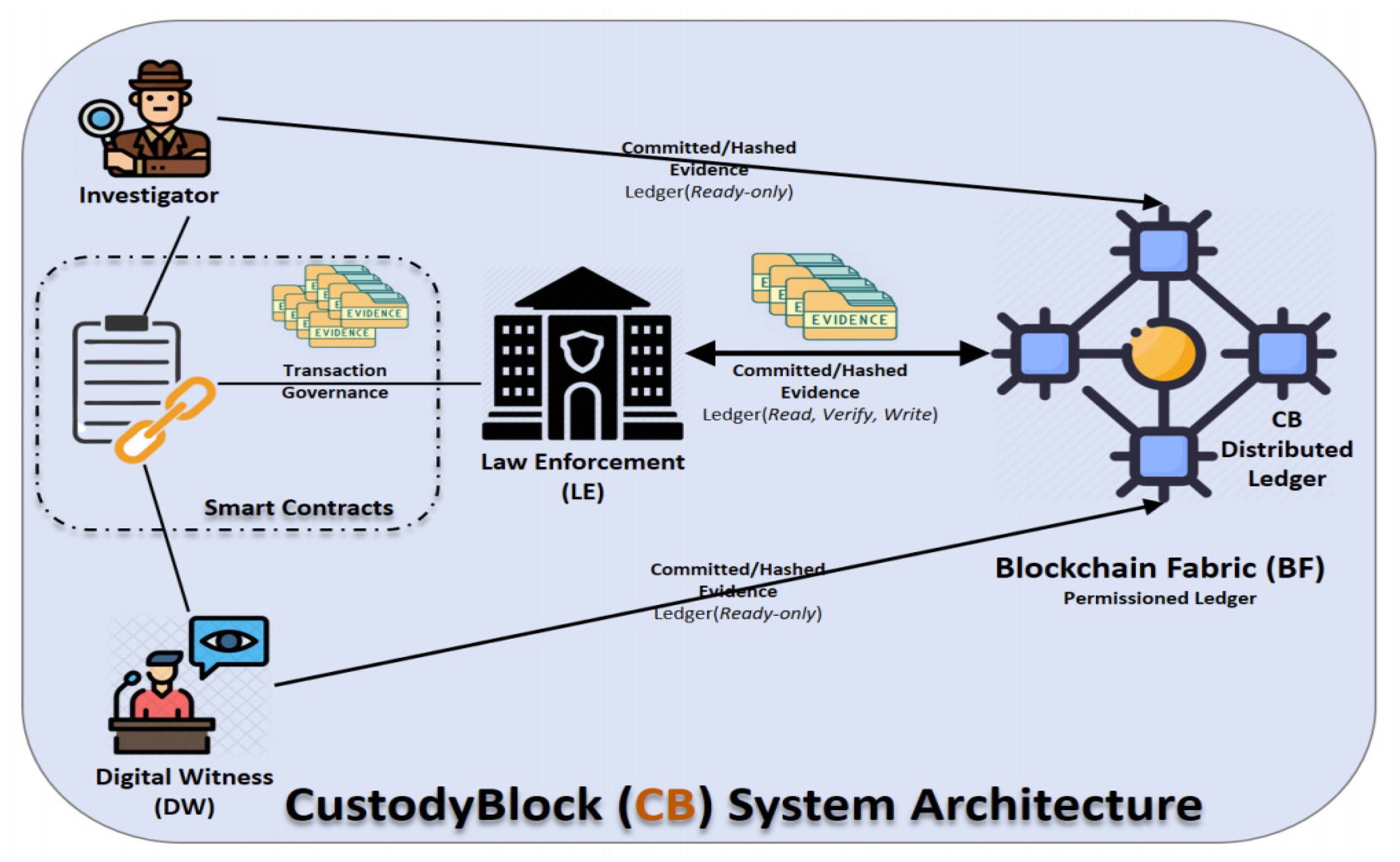
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig3 Alruwaili Information21 12-2.png|240px]]</div> | <div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig3 Alruwaili Information21 12-2.png|240px]]</div> | ||
'''"[[Journal:CustodyBlock: A distributed chain of custody evidence framework|CustodyBlock: A distributed chain of custody evidence framework]]"''' | '''"[[Journal:CustodyBlock: A distributed chain of custody evidence framework|CustodyBlock: A distributed chain of custody evidence framework]]"''' | ||
Latest revision as of 15:33, 2 January 2024
|
|
If you're looking for other "Article of the Week" archives: 2014 - 2015 - 2016 - 2017 - 2018 - 2019 - 2020 - 2021 - 2022 - 2023 - 2024 |
Featured article of the week archive - 2023
Welcome to the LIMSwiki 2023 archive for the Featured Article of the Week.
Featured article of the week: December 25–31:"Thirty years of the DICOM standard" Digital Imaging and Communications in Medicine (DICOM) is an international standard that defines a format for storing medical images and a protocol to enable and facilitate data communication among medical imaging systems. The DICOM standard has been instrumental in transforming the medical imaging world over the last three decades. Its adoption has been a significant experience for manufacturers, healthcare users, and research scientists. In this review, 30 years after introducing the standard, we discuss the innovation, advantages, and limitations of adopting DICOM and its possible future directions ... (Full article...)
|